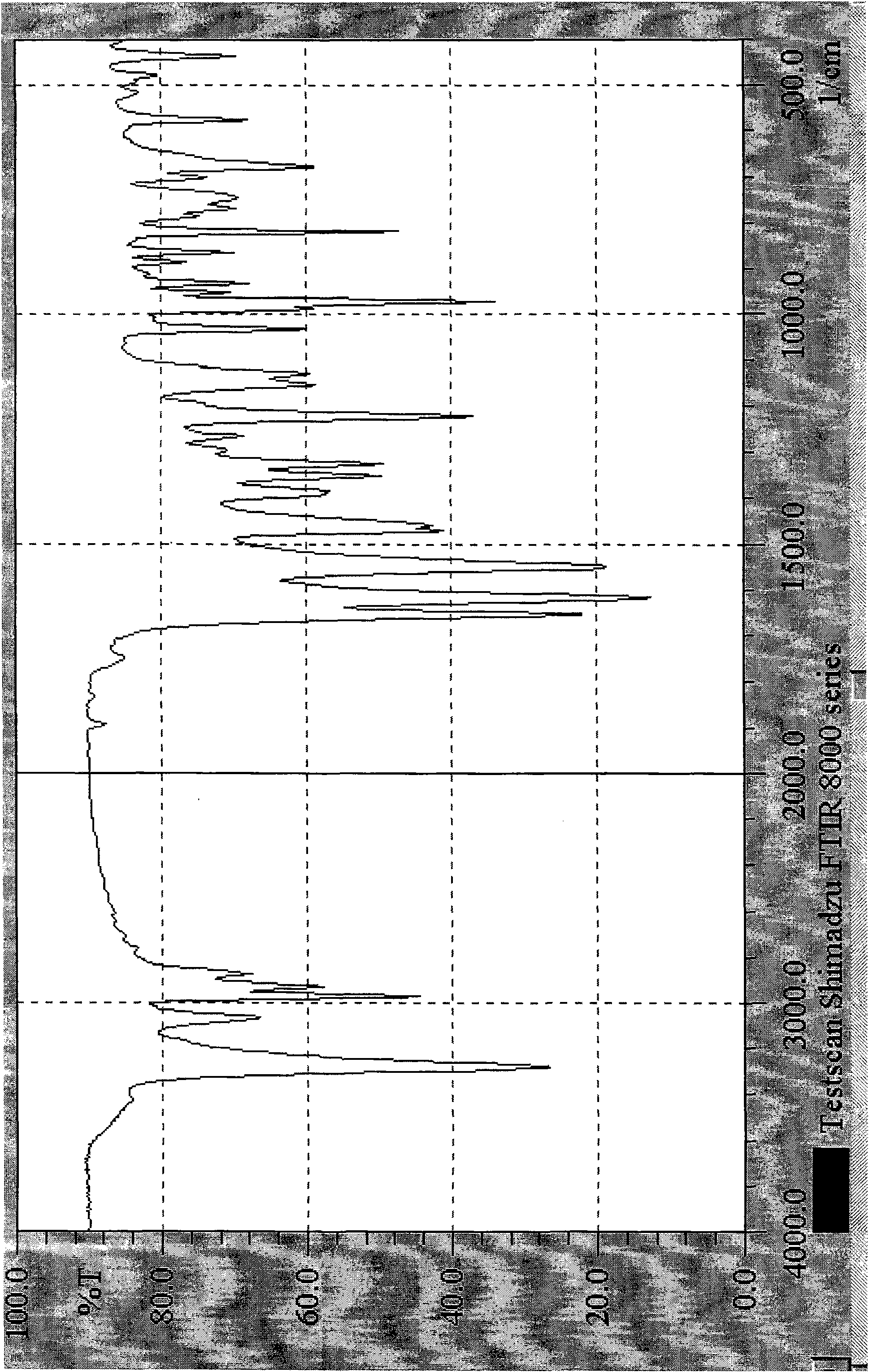
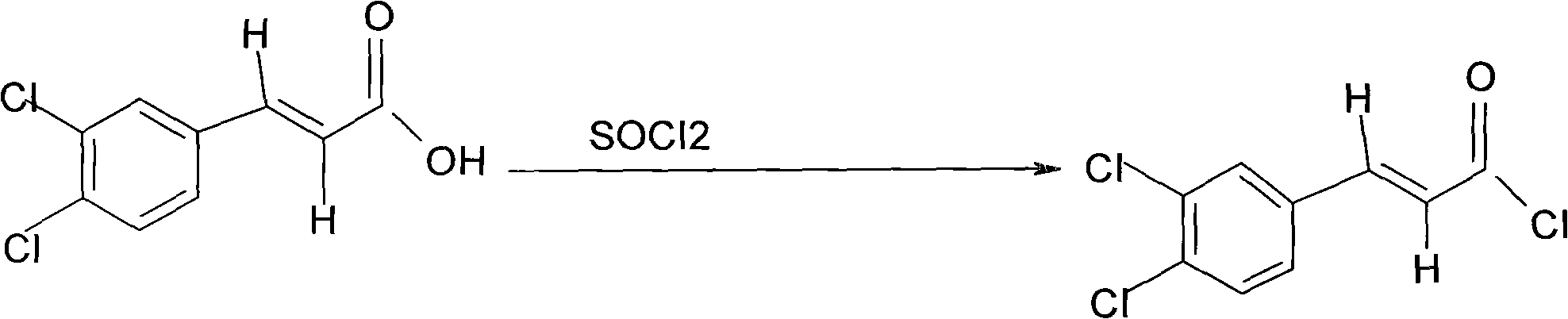Preparation method of chlocibutamine
A kind of technology of chlornamonamide and sec-butylamine, which is applied in the field of preparation of chlornamonamide, and can solve the problems such as the preparation method of compounds that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of 3,4-dichlorophenylacrylic acid
[0024] 2.5kg (14.28 moles) of 4-dichlorobenzaldehyde, 1.41kg (14.36 moles) of potassium acetate, 3.96kg (38.82 moles) of acetic anhydride, pyridine 0.19 kg (2.45 moles). 138 ~ 142 ℃ heat preservation reaction. After the reaction was completed, the reaction solution was slowly poured into 18 liters of water, stirred while pouring, cooled to 25°C with stirring, filtered under reduced pressure, washed with water, and dried to obtain crude 3,4-dichlorophenylacrylic acid.
[0025] Put the crude product of 3,4-dichlorophenylacrylic acid into the reactor, add 12 liters of water and 1.7 liters of concentrated ammonia water, heat and stir to dissolve. Add 0.2 kg of activated carbon and stir at 95°C for 1 hour. Filtrate hot, add concentrated hydrochloric acid to the filtrate in portions under stirring, control the pH at 2.0-2.5, a large amount of white precipitate precipitates, stir and cool down to 17°C. It was filtered under r...
Embodiment 2
[0031] Preparation of 3,4-dichlorophenylacrylic acid
[0032] In 10 liters of glass reactors with stirring and reflux, add 3,4-dichlorobenzaldehyde 2.5kg (14.28 moles) respectively, potassium acetate 1.68kg (17.14 moles), acetic anhydride 3.68kg (36.0 moles), Pyridine 0.169kg (2.61 moles). 135 ~ 138 ℃ insulation for 6 hours. The reaction solution was slowly poured into a stainless steel bucket filled with 16 liters of water, and stirred while pouring. A large amount of yellow precipitate was precipitated, stirred and cooled to 25°C, filtered under reduced pressure, washed with water, and dried to obtain 3,4-dichlorophenylacrylic acid.
[0033] Put the crude product of 3,4-dichlorophenylacrylic acid into the reactor, add 12 liters of water and 1.7 liters of concentrated ammonia water, heat and stir to dissolve. Add 0.2 kg of activated carbon and stir at 95°C for 1 hour. Filtrate hot, add concentrated hydrochloric acid to the filtrate in portions under stirring, control the ...
Embodiment 3
[0039] Preparation of 3,4-dichlorophenylacrylic acid
[0040] In 10 liters of glass reactors with stirring and reflux, add 3,4-dichlorobenzaldehyde 2.5kg (14.28 moles) respectively, potassium acetate 1.68kg (17.14 moles), acetic anhydride 3.64kg (35.7 moles), Pyridine 0.28kg (3.57 moles). Keep warm at 143-145°C. The reaction solution was slowly poured into a stainless steel bucket filled with 18 liters of water, and stirred while pouring. A large amount of yellow precipitate was precipitated, stirred and cooled to 25°C, filtered under reduced pressure, washed with water, and dried to obtain 3,4-dichlorophenylacrylic acid.
[0041] Put the crude product of 3,4-dichlorophenylacrylic acid into the reactor, add 10 liters of water and 1.5 liters of concentrated ammonia water, heat and stir to dissolve. Add 0.2 kg of activated carbon and stir at 95°C for 1 hour. Filtrate hot, add concentrated hydrochloric acid to the filtrate in portions under stirring, control the pH at 2.0-2.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com