Chinese medicinal composition and preparation for loosening bowel and relieving constipation and preparation method thereof
A technology of laxative and traditional Chinese medicine preparations, applied in the directions of drug combinations, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve problems such as aggravating constipation, physical weakness of the elderly, and achieve fast onset of effect and easy swallowing. , quantitative and accurate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of embodiment 1 medicine capsule of the present invention
[0054] Raw material and dosage:
[0055] Cistanche 20g, radish seed 15g, cassia seed 15g, Atractylodes macrocephala 20g.
[0056] Preparation:
[0057] (1) Weigh each crude drug according to the above-mentioned dosage, first extract Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizome, Semen Cassiae and Semen Radix with reflux of 10 times of ethanol twice for 1 hour and 2 hours respectively.
[0058] (2) Add the reflux extract of step (1) to anhydrous sodium sulfate for dehydration, leave it to stand overnight, filter (80 mesh sieve) to obtain volatile oil, and dregs for subsequent use;
[0059] (3) Add the dregs of step (2) into Cistanche cistanche, decoct and extract twice, the first time for 1 hour, and the second time for 2 hours, the amount of water added is 10 times the amount, and the medicinal liquid obtained from the two extractions is mixed and spray-dried to obtain dry po...
Embodiment 2
[0062] Embodiment 2 capsule preparation of the present invention
[0063] Raw material and dosage:
[0064] Cistanche 10g, radish seed 20g, cassia seed 20g, Atractylodes macrocephala 10g.
[0065] Preparation:
[0066] (1) Weigh each crude drug according to the above-mentioned dosage, first extract Atractylodes Rhizoma Atractylodes Rhizoma Atractylodes Rhizome, Semen Cassiae and Semen Radix with reflux of 10 times of ethanol twice for 1 hour and 2 hours respectively.
[0067] (2) Add the reflux extract of step (1) to anhydrous sodium sulfate for dehydration, leave it to stand overnight, filter (80 mesh sieve) to obtain volatile oil, and dregs for subsequent use;
[0068] (3) Add the dregs of step (2) into Cistanche cistanche, decoct and extract twice, the first time for 1 hour, and the second time for 2 hours, the amount of water added is 10 times the amount, and the medicinal liquid obtained from the two extractions is mixed and spray-dried to obtain dry powder for use;
...
Embodiment 3
[0071] Embodiment 3 Preparation of pharmaceutical granules of the present invention
[0072] Preparation:
[0073] Weigh the crude drug according to the following dosage, soak the crude drug of the present invention in water for 20-30 minutes according to the conventional method, add 3 times the amount of water to boil and keep boiling for 10-15 minutes, take the medicinal liquid; add 3 times the amount of water Keep boiling for 10-15 minutes after decocting, take the liquid medicine; mix and filter the two liquid medicines, concentrate to a thick paste with a relative density of 1.3-1.34, spray granulate, and dry. Subpackage.
[0074] Raw material and dosage:
[0075] Cistanche 25g, radish seed 10g, cassia seed 10g, Atractylodes macrocephala 25g.
[0076] Clinical experiment of the present invention and result analysis:
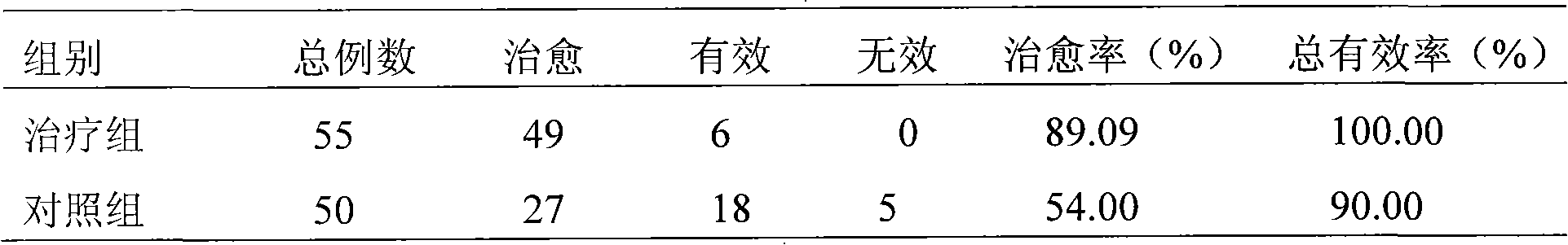
[0077] Case: A patient with constipation over the age of 55 was selected; the course of the disease was over 1 year; the stool was dry and hard to defec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com