Medicament for treating digestive tract side effects caused by cancer chemotherapy
A technology for digestive tract and side effects, applied in the field of traditional Chinese medicine, can solve the problems such as the lack of Chinese patent medicines that have not yet entered the system development stage, and achieve the effects of reducing animal toxic and side effects, delaying the occurrence of vomiting, and reducing the number of vomiting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] The original medicinal materials include: Astragalus 700g, Ophiopogon 260g, Red ginseng 230g, Atractylodes macrocephala 250g, Poria 200g, Licorice 220g, Scrophulariaceae 230g, Ebony 300g, Curcuma 250g, Angelica 200g, Agastache 220g, Tangerine peel 230g, French Pinellia 300g.
[0011] Effect on the phagocytic function of mouse macrophages: 50 mice weighing 18-22 grams, half male and half female, were randomly divided into blank control group, high, medium and low dose groups and Shenqi tablets group, each group of mice Gavage according to the dosage in Table 1, once a day for 7 consecutive days. The blank control group was given the same volume of distilled water, 1 hour after the last administration, according to the literature According to the method described in the method, the mice were injected with 5% chicken erythrocyte normal saline suspension 0.5ml / mouse, 3 hours later, the cervical spine was pulled to kill the mouse, the back was fixed, and 2ml / mouse normal saline ...
Embodiment 2
[0034] The original medicinal materials include: Astragalus 750g, Ophiopogon 280g, Red ginseng 250g, Atractylodes macrocephala 200g, Poria 220g, Licorice 230g, Scrophulariaceae 250g, Black plum 250g, Curcuma turmeric 200g, Angelica 220g, Agastache 230g, Tangerine peel 250g, French pinellia 250g.
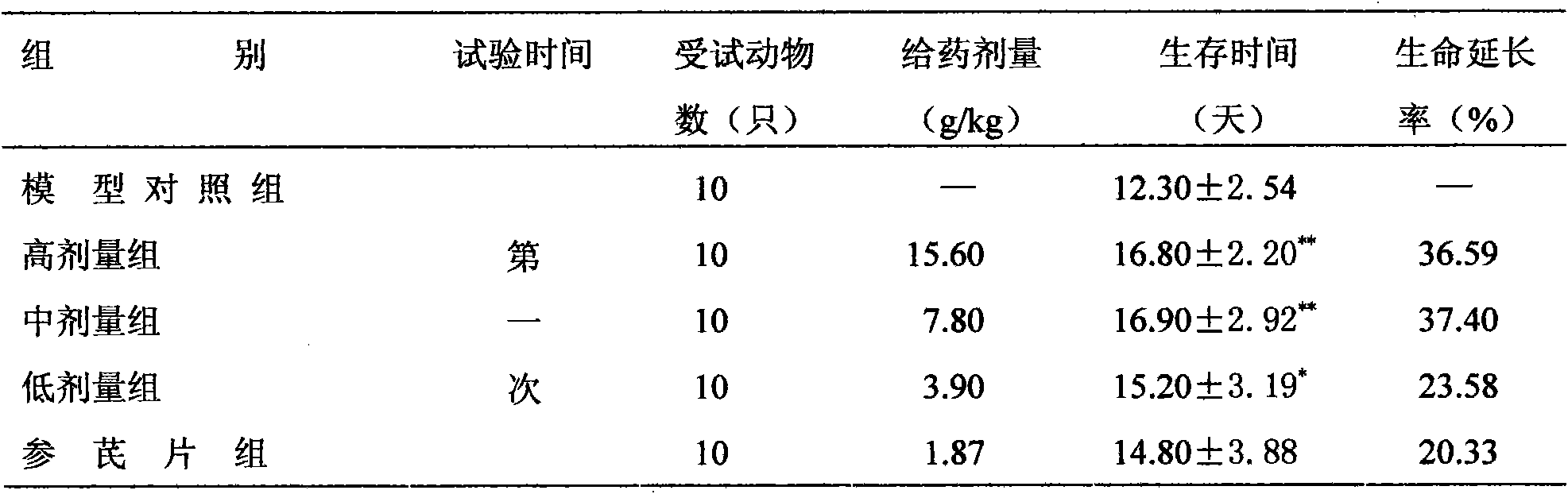
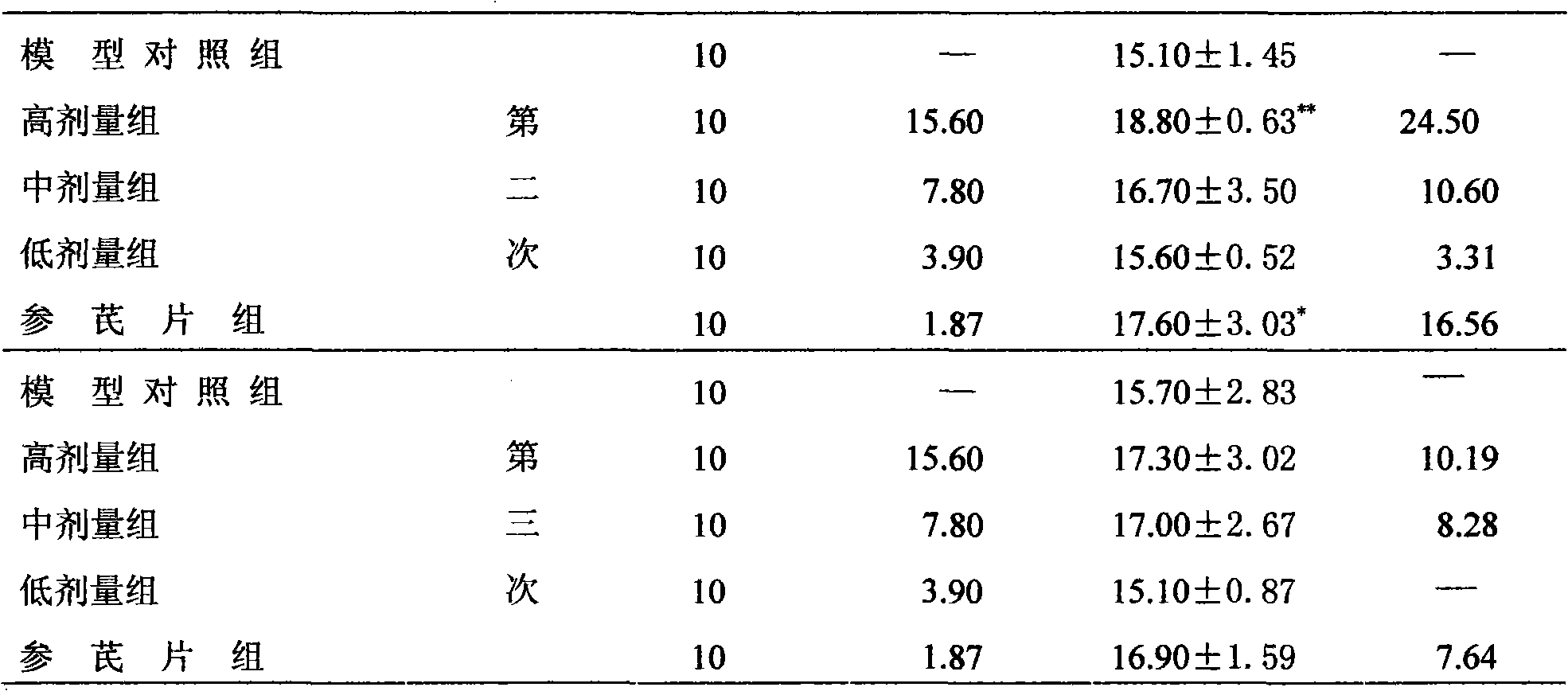
[0035] Effect on the toxicity and side effects of cyclophosphamide in mice: Take 60 mice weighing 18-22g, male and female, and randomly divided into model control group, high, medium and low dose groups, cyclophosphamide group and Shenqi tablets group. Except for the cyclophosphamide group, each group was administered intragastrically according to the dosage in Table 5, once a day for 7 consecutive days. The model control group was given the same volume of distilled water. From the 5th day of administration, except for the model control group, the cyclophosphamide group and each administration group were intraperitoneally injected with cyclophosphamide injection at a dose of 100 mg / kg / d...
Embodiment 3
[0059] The original medicinal materials include: Astragalus 770g, Ophiopogon japonicus 7300g, Red ginseng 200g, Atractylodes macrocephala 220g, Poria 230g, Licorice 250g, Scrophulariaceae 200g, Ebony 280g, Curcuma turmeric 220g, Angelica 230g, Agastache 200~250g, Tangerine peel 200g, French pinellia 260g.
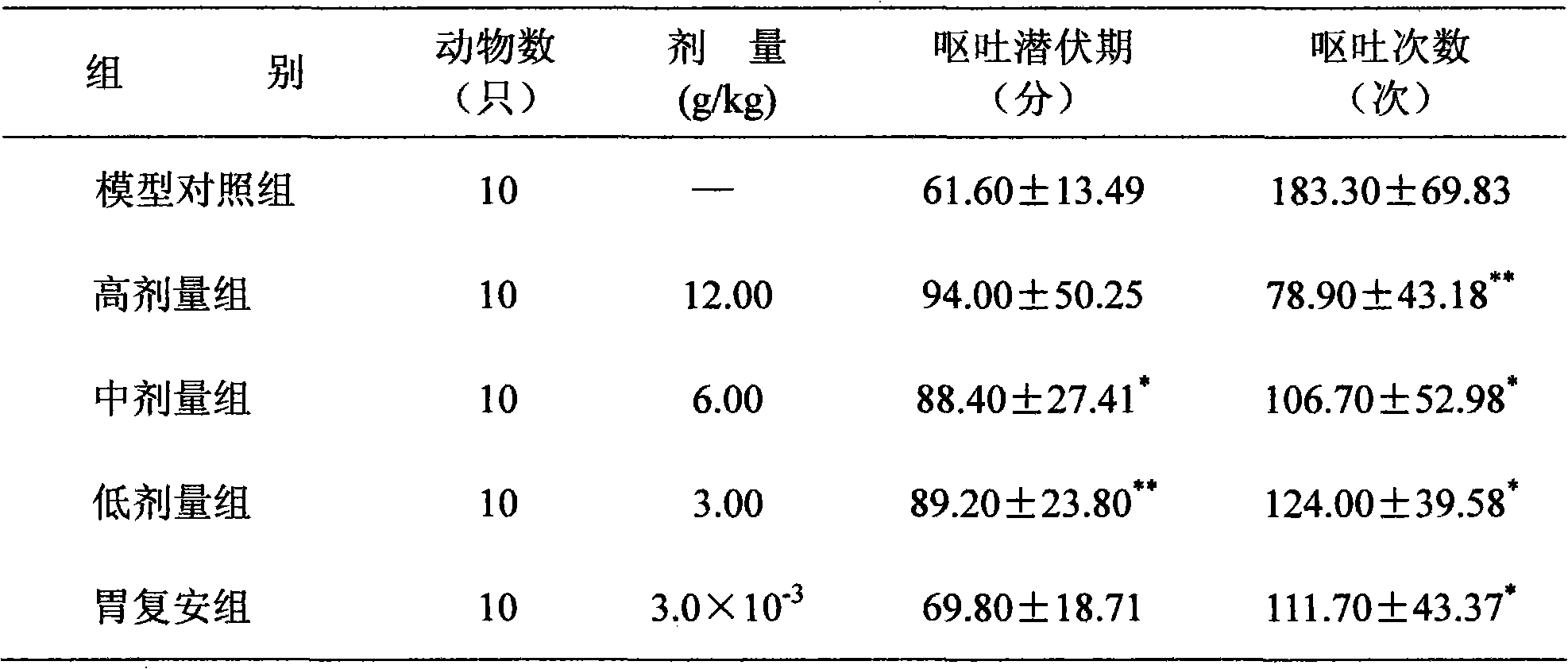
[0060] To S 180 Experiment on the effect of tumor weight on mice: Take 50 mice weighing 18-22g, half male and half, under aseptic conditions, routinely inoculate S under the armpit of the right forelimb of the mice. 180 Mouse abdominal cavity tumor fluid 0.2ml / mouse, on the second day after inoculation, randomly divided into high, medium and low dose groups, Shenqi tablets group and model control group according to body weight. The mice in each group were given intragastric administration according to the dosage in Table 9, once a day for 7 consecutive days. The model control group was given the same volume of distilled water. The mice were killed and weighed the next day afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com