Application of iridoid compound to preparation of ovarian cancer resistance medicament
A technology of iridoids and iridoids, which is applied in the field of application of iridoids in the preparation of anti-ovarian cancer drugs, and can solve the problems of insufficient research and chemical composition research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
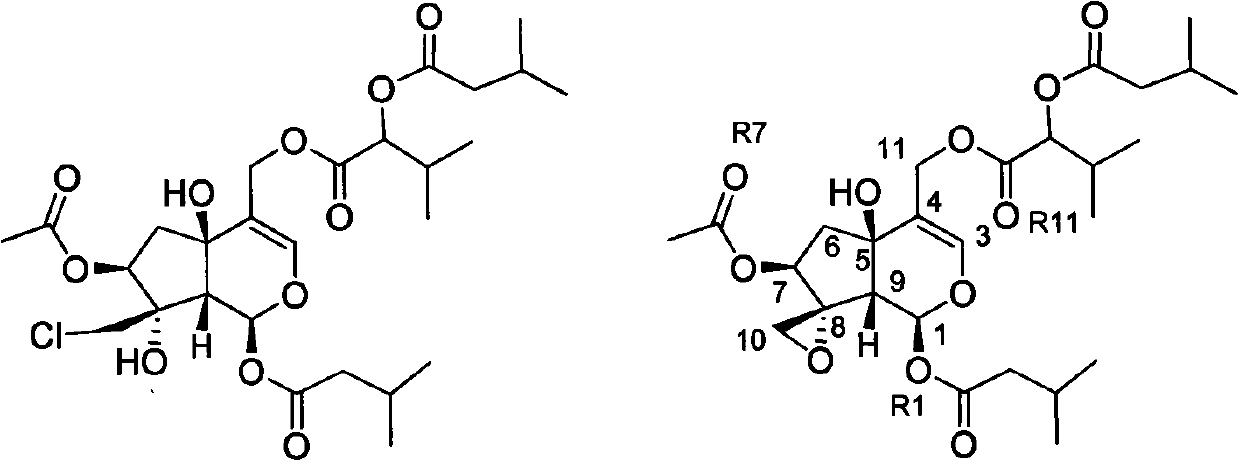
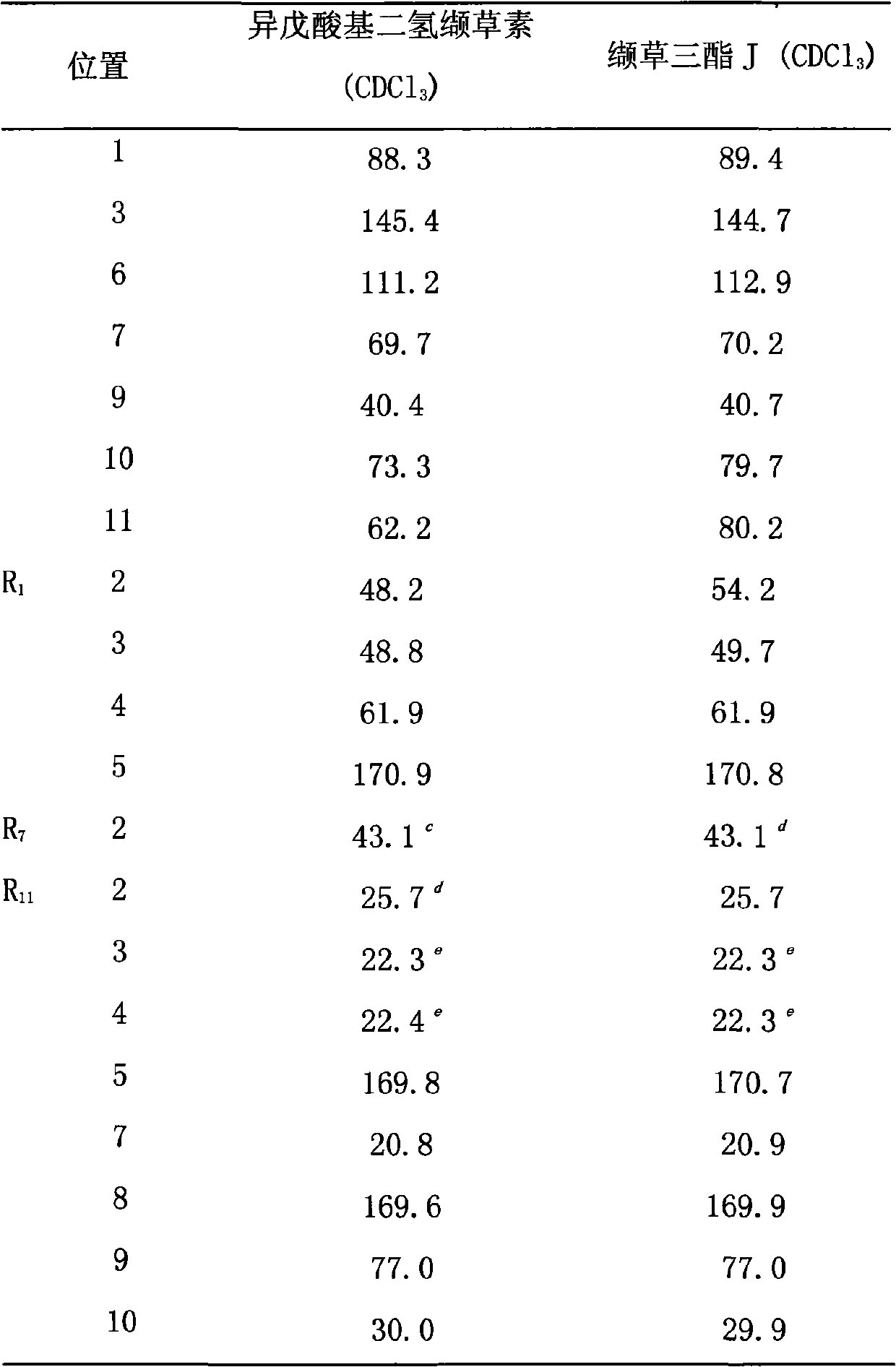
[0025] The preparation of embodiment 1 isovaleryl dihydrovalerenin and valerian triester J
[0026] 8.5Kg of the dried Herba chinensis was crushed and extracted by percolation with 95% ethanol for 4 days to obtain 100 L of extract. The extract was concentrated under reduced pressure into an extract, suspended in water, and extracted 5 times with ethyl acetate, 3 L each time. After the solvent was recovered under reduced pressure, the ethyl acetate part was 420 g, and the water phase part was 315 g. The ethyl acetate part was separated by normal-phase silica gel (1500g) column chromatography, and gradient elution was carried out with petroleum ether-acetone (100:0-0:100), and the eluent was detected by thin-layer chromatography, and the eluents of similar composition were combined , and the solvent was recovered to obtain 1-×10 (the eluate was divided into 10 elution fractions according to the different spots detected by the thin layer). The 4th elution fraction was taken and ...
Embodiment 2
[0034] Example 2 The cytotoxic effect of isovaleryl dihydrovalerenin and valerian triester J on ovarian cancer cells
[0035] Isovaleryl dihydrovalerenin and valerian triester J compared the toxic killing effects of ovarian cancer cells A2780 (p53 wild type), OVCAR-3 (p53 mutant) and ovarian normal cells IOSE144.
[0036] Isovaleryl dihydrovalerenin and valerian triester J experimental drug treatment time is 48h; drug concentration is set to 0, 1, 5, 10, 50, 100 μ M; drug dissolved in DMSO (1000 ×); drug treatment When the content of DMSO is less than 0.1%.
[0037] The results of the study are shown in Table 3. Isovaleryl dihydrovalerian and valerian triester J The toxic killing effect of IOSE144 on ovarian normal cells is less than that of ovarian cancer cell lines, indicating that isovaleryl dihydrovalerian and valerian triester J has selective toxic killing effect on ovarian cancer cells.
[0038] Table 3 IC50 values of isovaleryl dihydrovalerenin and valerian trieste...
Embodiment 3
[0040] Example 3 Effects of isovaleryl dihydrovalerian and valerian triester J on the proliferation of ovarian cancer cell line A2780, OVCAR-3 and normal ovarian cell line IOSE144
[0041]Isovaleryl dihydrovalerenin and valerian triester J have selective toxic and killing effects on ovarian cancer cells (compared to normal ovarian cell lines). The study explored the effects of these two compounds on ovarian cancer cell lines A2780, 3 and the effect on the proliferation of normal ovarian cell line IOSE144.
[0042] Experimental time gradient: 0, 24, 48, 72 hours; isovaleryl dihydrovalerian and valerian triester J experimental drug concentration gradient settings: 0, 1, 5, 10, 25, 50, 100 μM; drug Soluble in DMSO (1000×); the content of DMSO is less than 0.1% when the drug is treated.
[0043] Experimental method: MTT assay; cell viability was detected every 24 hours.
[0044] The results of the study showed that isovaleryl dihydrovalerian and valerian triester J can significa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com