N-(ethylamino) inulin and preparation and application thereof
A technology of ethyl amino and inulin, which is applied in the field of daily chemicals, can solve the problems of few renewable resources and achieve the effects of low cost, improved reactivity, and high degree of substitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
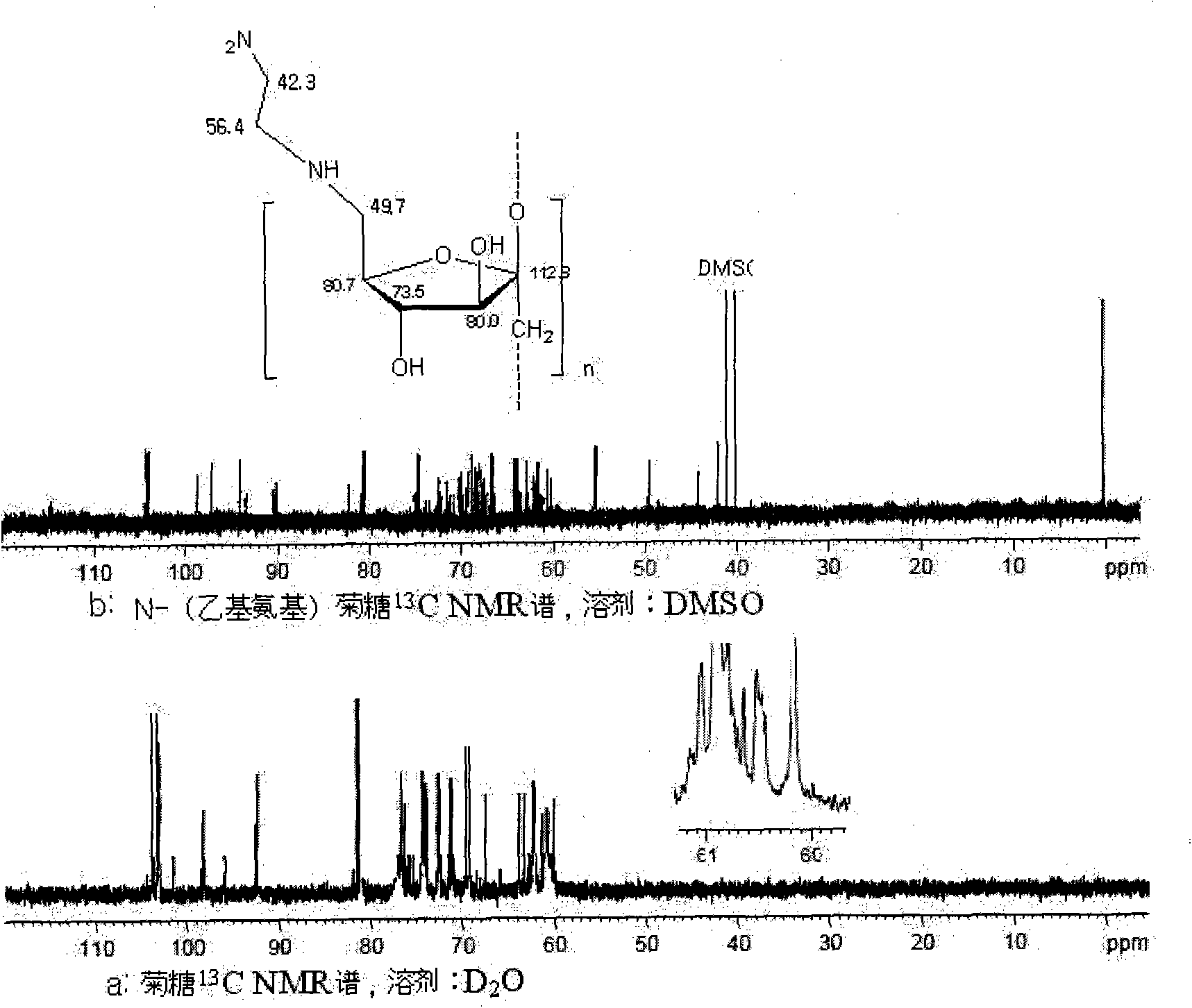
[0020] N-(ethylamino) inulin is a compound represented by formula (1).
[0021]
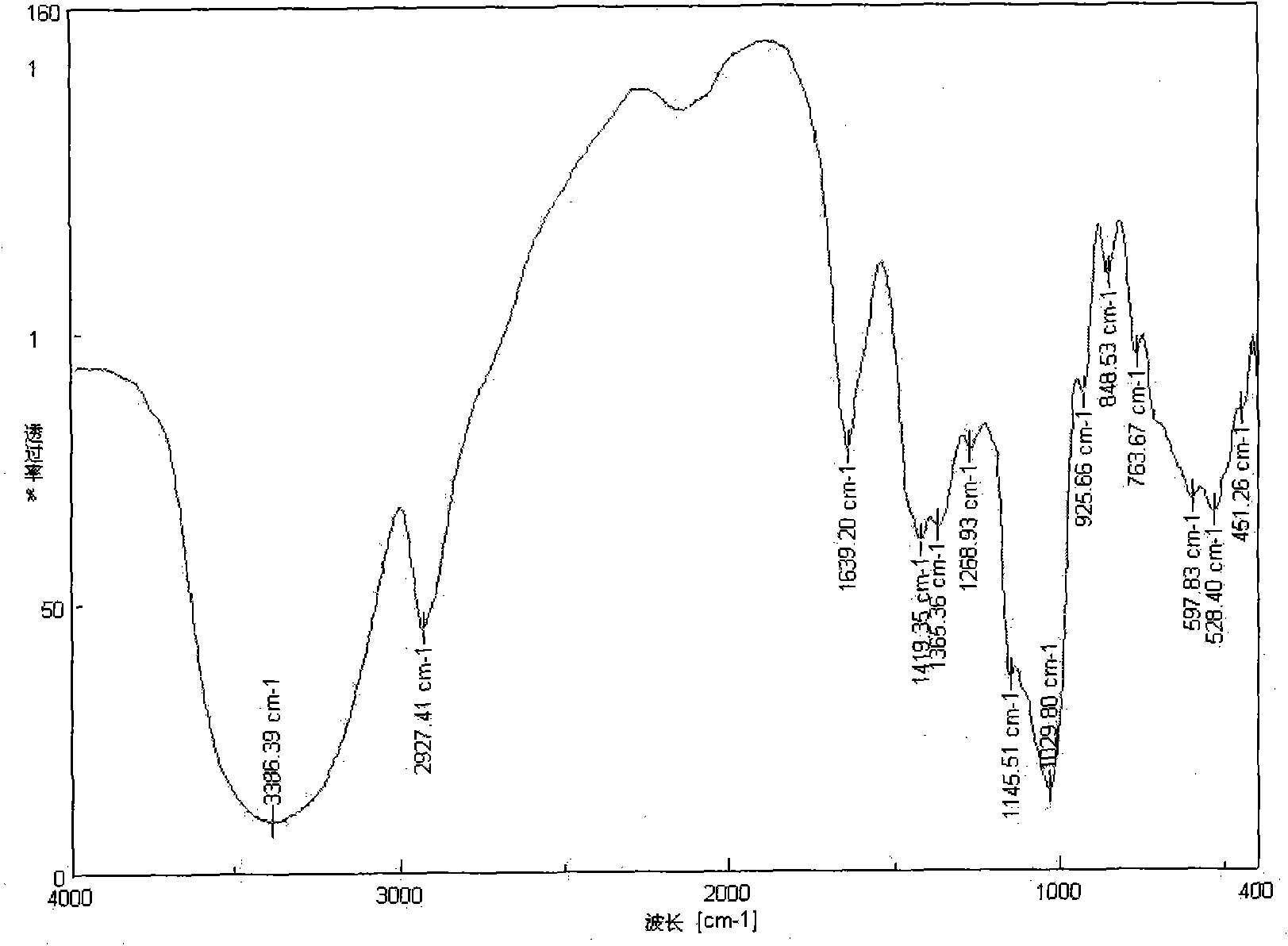
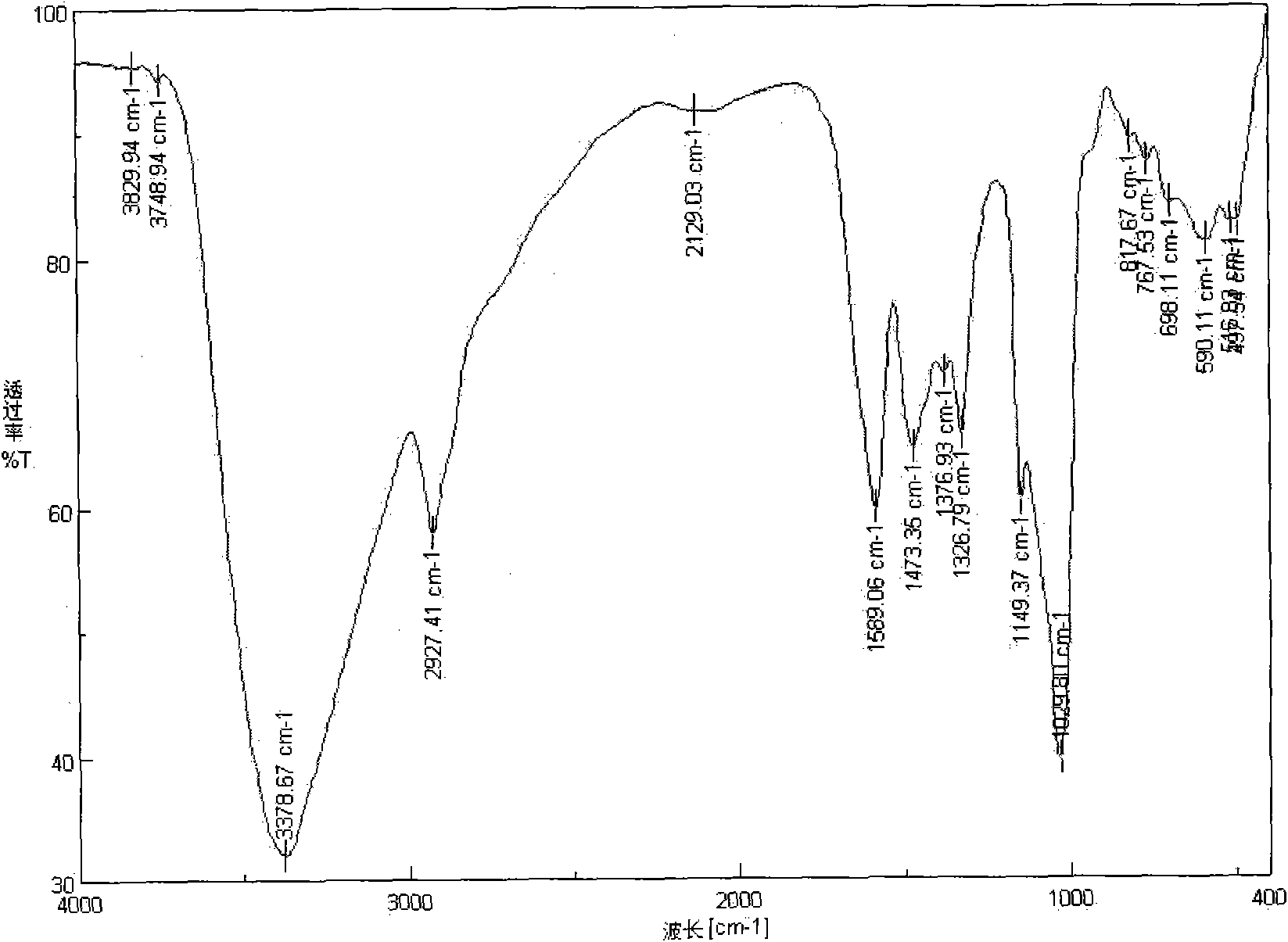
[0022] Preparation of N-(ethylamino)inulin: 1.62g of inulin and 1.90g of p-toluenesulfonyl chloride were dissolved in 30mL of nitrogen dimethylformamide, stirred and reacted at room temperature for 10h. After the reaction was finished, the reaction solution was poured into 150 mL of acetone, and a precipitate was precipitated. The precipitate was washed with acetone and tetrahydrofuran, then vacuum freeze-dried, and set aside. The produced product was p-tosylated inulin, 3.2g was obtained, which was dissolved in 22mL of anhydrous ethylenediamine, and reacted at 50°C for 12h. After the reaction was completed, the product was precipitated with 100 mL of acetone, washed with ether and acetone respectively, and vacuum-dried at -50°C to obtain the product. The resulting product is a milky white powder, easily soluble in water, and its infrared spectrogram is shown in figure 1 with figure 2 , w...
Embodiment 2
[0025] The difference from Example 1 is:
[0026] (1) Inulin and lithium bromide were dried under vacuum at 100°C for 8 hours. Take 1.62g of inulin and 0.86g of lithium bromide and add them to 20mL of purified nitrogen-nitrogen dimethylformamide under the protection of nitrogen, heat up until inulin and lithium bromide are completely dissolved, and add 3.5g of N -Bromosuccinimide (NBS) was added to the above reaction solution. Weigh 5.2 g of triphenylphosphine and dissolve it in 10 mL of nitrogen-nitrogen dimethylformamide. This solution was added dropwise to the reaction solution at room temperature. After the reaction solution was reacted at room temperature for 30 minutes, the temperature of the reaction system was raised to 70°C. After the reaction was carried out at 70°C for 3 hours, the reaction solution was poured into 150mL acetone, and a precipitate was precipitated. The precipitate was suction filtered, washed with acetone and tetrahydrofuran, and vacuum freeze-dri...
Embodiment 3
[0029] The difference from Example 1 is that: 1.62g of inulin and 1.90g of p-toluenesulfonyl chloride were dissolved in 30mL of nitrogen-nitrogen dimethylformamide, and the reaction was stirred at room temperature for 6h under the catalysis of 3.7mL of triethylamine. After the reaction was finished, the reaction solution was poured into 150 mL of acetone, and a precipitate was precipitated. The precipitate was washed with acetone and tetrahydrofuran, and then vacuum freeze-dried to obtain 3.0 g of tosylated inulin. The prepared product was dissolved in 27mL of anhydrous ethylenediamine, and reacted at 70°C for 10h. After the reaction was completed, the product was precipitated with 100 mL of acetone, washed with ether and acetone respectively, and vacuum-dried at -50°C to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com