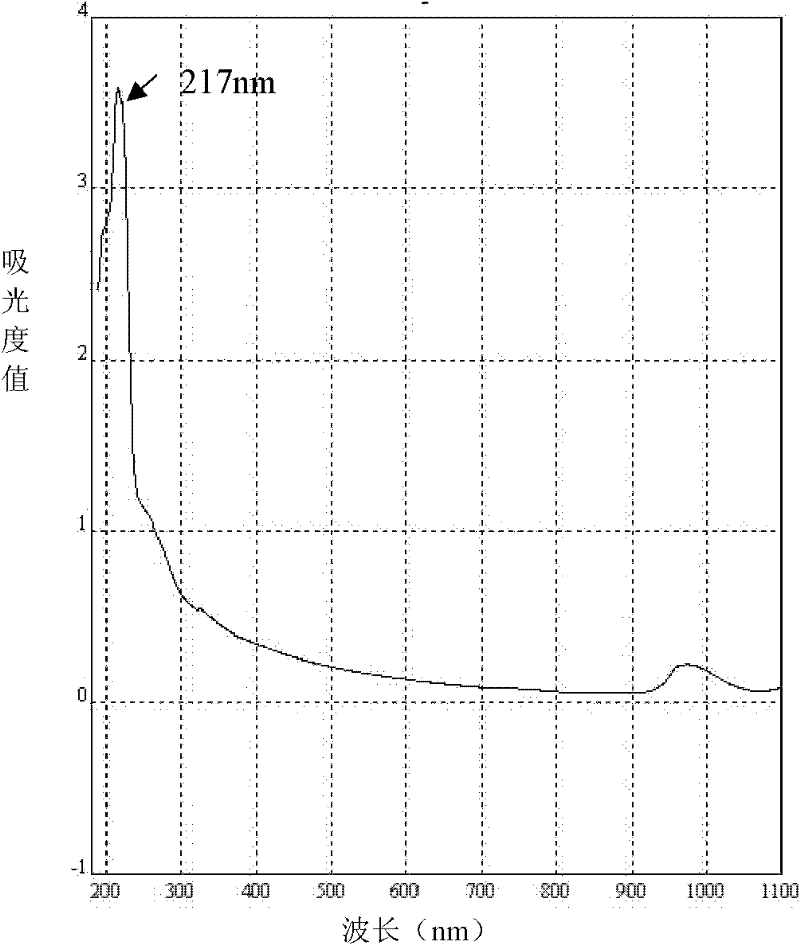
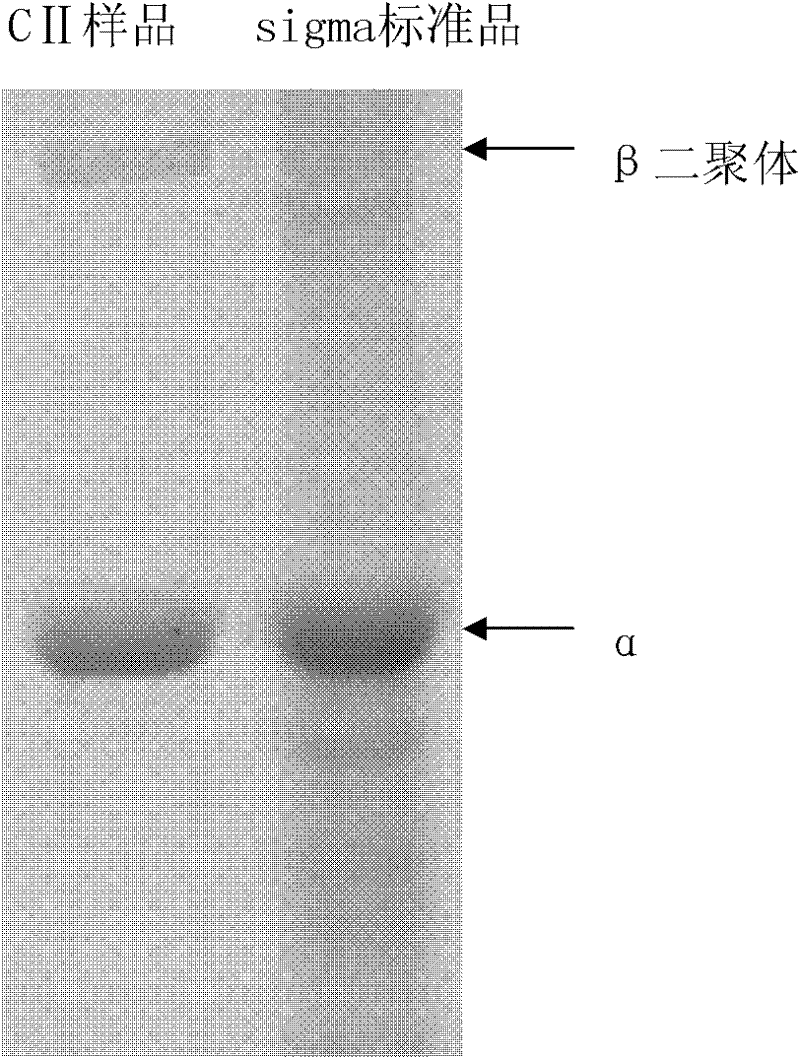
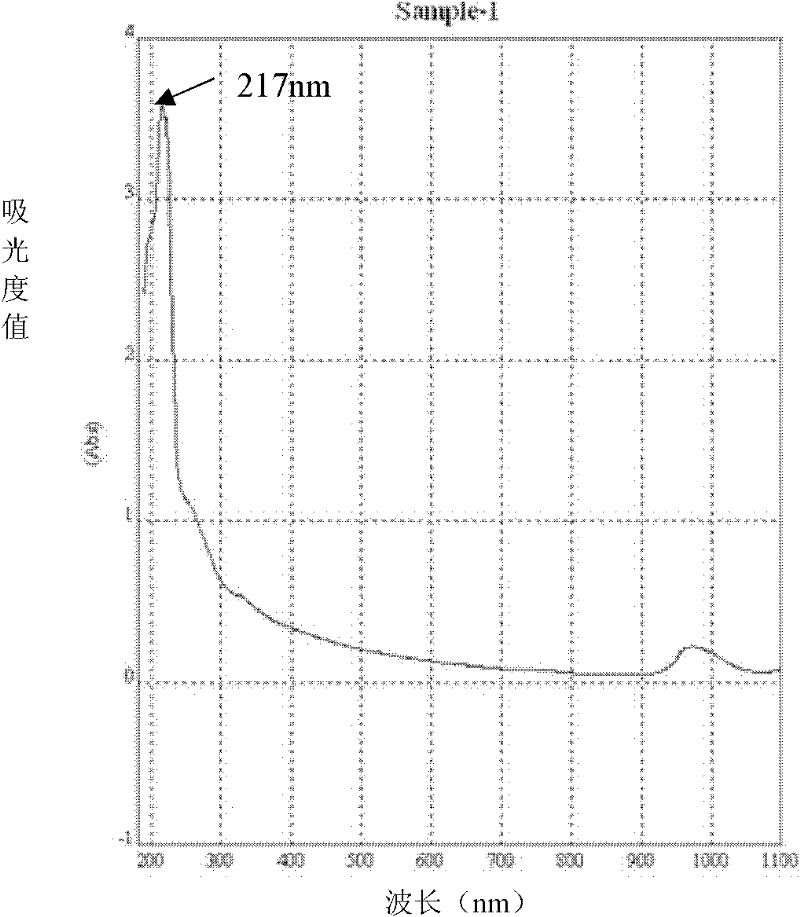
II-type collagen joint cartilage fluid and preparation method thereof
A technology of articular cartilage and repair fluid, which is applied to bone diseases, medical formulas, medical preparations with non-active ingredients, etc., and can solve the problem of non-toxic, sterile, non-pyrogenic Type II collagen joint repair fluid research reports, etc. problem, achieve the effect of improving joint movement function, preventing aggravation of the disease, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preferred embodiment of the non-toxic, non-pyrogenic and highly active type II collagen preparation method of the present invention is:
[0033] (1) Raw material handling
[0034] The hyaline cartilage of pig joints is removed through fascial fat removal→cleaning and soaking→toxic substance treatment→disinfection to remove all bacteria, viruses and other microorganisms.
[0035] The specific method process is as follows:
[0036] The fascial adipose tissue in the porcine articular hyaline cartilage was completely removed with a blade, and sliced into thin slices. Degrease and serum components with 20% NaCl+0.05M Tris (pH7.5), wash thoroughly until no lipid floats, and the liquid has no foam. After being treated with 0.5% chlorhexidine by volume at room temperature for 30 minutes, it was fully washed. Treat with 0.1% peracetic acid by volume at room temperature for 15 minutes, wash and pack into sealed bags and send to Co 60 Sterilize by irradiation with a dose o...
Embodiment 2
[0065] A preferred embodiment of the non-toxic, non-pyrogenic and highly active type II collagen preparation method of the present invention is:
[0066] (1) Raw material handling
[0067] The hyaline cartilage of pig joints is removed through fascial fat removal→cleaning and soaking→toxic substance treatment→disinfection to remove all bacteria, viruses and other microorganisms.
[0068] The specific method process is as follows:
[0069] The fascial adipose tissue in the porcine articular hyaline cartilage was completely removed with a blade, and sliced into thin slices. Degrease and serum components with 20% NaCl+0.05M Tris (pH7.5), wash thoroughly until no lipid floats, and the liquid has no foam. After treating with 0.1% chlorhexidine at room temperature for 60 minutes by volume percentage, fully wash. Treat with 1% peracetic acid by volume at room temperature for 20 minutes, wash and pack into sealed bags, then send to Co 60 Sterilize by irradiation with a dose of 3...
Embodiment 3
[0098] A preferred embodiment of the non-toxic, non-pyrogenic and highly active type II collagen preparation method of the present invention is:
[0099] (1) Raw material handling
[0100] The hyaline cartilage of pig joints is removed through fascial fat removal→cleaning and soaking→toxic substance treatment→disinfection to remove all bacteria, viruses and other microorganisms.
[0101] The specific method process is as follows:
[0102] The fascial adipose tissue in the porcine articular hyaline cartilage was completely removed with a blade, and sliced into thin slices. Degrease and serum components with 20% NaCl+0.05M Tris (pH7.5), wash thoroughly until no lipid floats, and the liquid has no foam. After being treated with 5% chlorhexidine at room temperature for 10 minutes by volume, it was fully washed. Treat with 3% peracetic acid by volume at room temperature for 5 minutes, wash and pack into sealed bags, then send to Co 60 Sterilize by irradiation with a dose of 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com