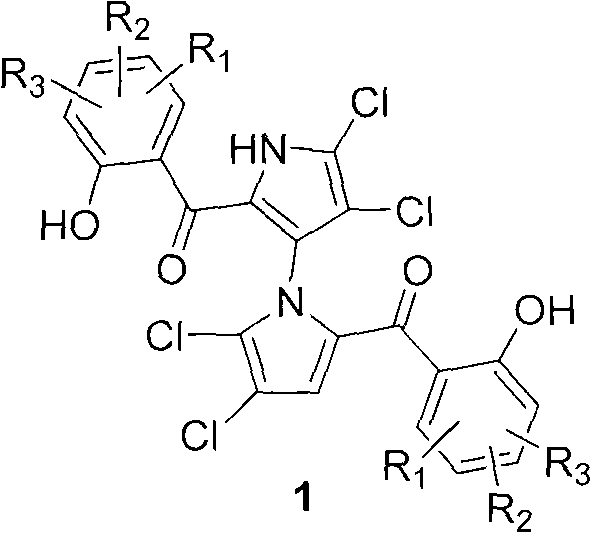
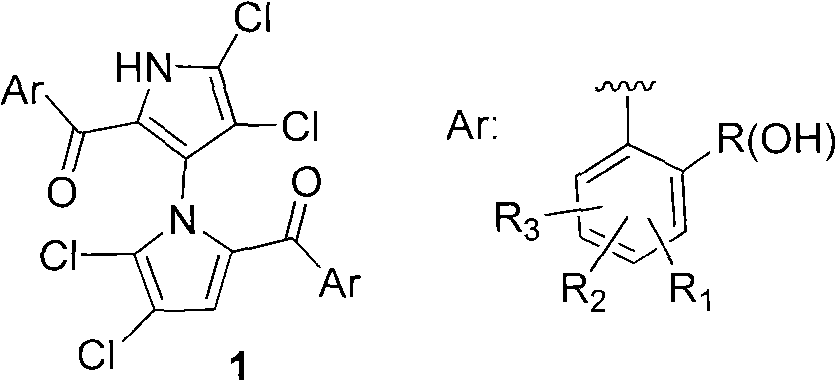
(+/-)-marinopyrrole A for resisting methicillin-resistant staphylococcus aureus (MRSA) and synthesized derivative thereof
A compound, methoxy technology, applied in the field of synthetic derivatives of natural product-marinopyrrole A, can solve problems such as synthesis and structure-activity relationship research reports, and achieve the effect of short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Implementation Example 1 Preparation of Compound 5
[0054]
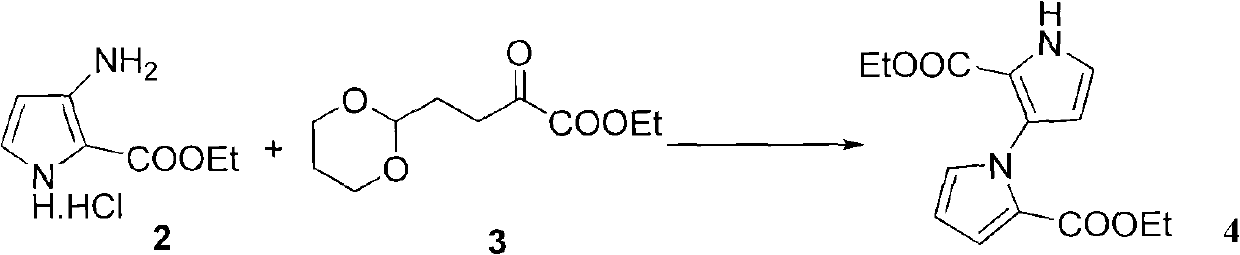
[0055] Compound 2 (2.00 g, 10.50 mmol) was dissolved in 20 mL of toluene, and compound 3 (3.40 g, 15.74 mmol) and p-toluenesulfonic acid (26 mg, 0.14 mmol) were added in sequence. The reaction solution was refluxed for 10 h, cooled to room temperature, adjusted to pH=7 with saturated sodium bicarbonate solution, extracted the aqueous phase (3×20 mL) with ethyl acetate, combined the organic phases, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure to obtain the crude product Separation and purification by silica gel column (10% ethyl acetate / petroleum ether) gave light yellow powdery solid 4 (2.40 g, yield 82%). Mp 70-71.6°C; 1 H NMR (CDCl 3 , 400MHz) δ1.11(t, J=7.2Hz, 3H), 1.23(t, J=7.2Hz, 3H), 4.10-4.19(m, 4H), 6.26(dd, J=4.0, 2.8Hz, 1H ), 6.31(t, J=2.8Hz, 1H), 6.89(t, J=1.6Hz, 1H), 6.91(t, J=3.2Hz, 1H), 7.07(dd, J=4.0, 2.0Hz, 1H ), 9.32 (br, s, 1H) ppm; 13 C NMR (C...
Embodiment 2
[0056] Implementation Example 2 Preparation of Compound 5
[0057]
[0058] Compound 4 (2.00 g, 7.25 mmol) was dissolved in 20 mL of dry CH 2 Cl 2 , DMAP (4.40g, 36.07mmol) and DIPEA (4.70g, 36.43mmol) were added at 0°C, and p-toluenesulfonyl chloride (11.50g, 72.33mmol) was slowly added after stirring for 10min, and the reaction solution rose to room temperature. After reacting for 8 h, the reaction solution was poured into water, and the 2 Cl 2 The aqueous phase was extracted (3×25 mL), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure. The crude product was separated and purified on a silica gel column (5% ethyl acetate / petroleum ether) to obtain a pale yellow solid 5 (2.96 g, yield 95%). Mp 87-89°C; 1 H NMR (400MHz, CDCl 3 )δ0.91(t, J=7.2Hz, 3H), 0.94(t, J=7.2Hz, 3H), 2.44(s, 3H), 3.97(q, J=7.2Hz, 2H), 4.07(q, J=7.2Hz, 2H), 6.22(dd, J=4, 2.8Hz, 1H), 6.37(d, J=3.6Hz, 1H), 6.81(dd,...
Embodiment 3
[0059] Implementation Example 3 Preparation of Compound 6
[0060]
[0061] In an ice-water bath, under nitrogen protection, DIBAL (4.67mL, 4.70mmol) was slowly added dropwise to compound 5 (500mg, 1.16mmol) in dry CH 2 Cl 2 (5 mL) solution. After the dropwise addition, the reaction solution was raised to room temperature to continue the reaction for 6 h, and then the solution was washed with saturated Na 2 SO 4 The solution quenched the reaction and a large amount of white precipitate formed. The precipitate was removed by filtration, the filter cake was washed with ethyl acetate (3×50 mL), the filtrates were combined, the solvent was evaporated under reduced pressure, and the crude product was separated and purified on a silica gel column (33% ethyl acetate / petroleum ether) to obtain a white solid 6 (370 mg, yield 92%). Mp 103-106°C; 1 H NMR (400MHz, CDCl 3 )δ2.44(s, 3H), 2.63(br, s, 1H), 3.48(br, s, 1H), 4.31(s, 2H), 4.42(s, 2H), 6.19(t, J=3.6Hz , 1H), 6.29(dd, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com