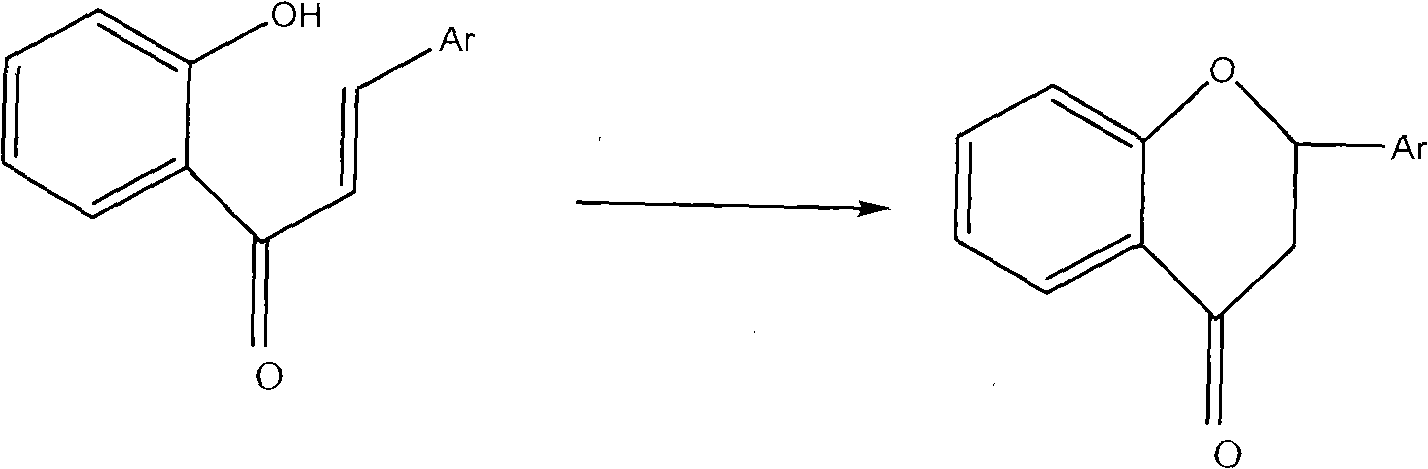
Method for preparing flavanone compound
A compound and flavanone technology, applied in the field of preparing flavanone compounds, can solve the problems of long reaction time, low yield and the like, and achieve the effects of short reaction time, simple operation, great application value and economic benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis of flavanones based on bromo-n-butylpyridine
[0033] In a 50ml beaker, add 2'-hydroxychalcone 10mg, brominated n-butylpyridine (0.015g / ml) 0.05ml, NaOH (8mol / L) 0.05ml and H 2 O 10ml, stirred vigorously, controlled the temperature at 15°C, and reacted for 1.5h; the reacted mixture was extracted with benzene, then concentrated under reduced pressure; The peak area was measured at the peak 251nm, and the flavanone content was calculated, and the yield was 59.1%.
Embodiment 2
[0034] Example 2: Synthesis of flavanones based on bromo-n-butylpyridine
[0035] In a 50ml beaker, add 2'-hydroxychalcone 10mg, brominated n-butylpyridine (0.015g / ml) 0.10ml, NaOH (8mol / L) 0.10ml and H 2 O 10ml, stirred vigorously, the temperature was controlled at 20°C, and reacted for 2h; the reacted mixture was washed with CH 2 Cl 2 Extraction is carried out, and then concentrated under reduced pressure; after the concentrate is chromatographically separated, the peak area is measured at the characteristic peak of flavanones at 251 nm by high performance liquid chromatography, and the content of flavanones is calculated, and the yield is 89.4%.
Embodiment 3
[0036] Example 3: Synthesis of flavanones based on bromo-n-butylpyridine
[0037] In a 50ml beaker, add 2'-hydroxychalcone 10mg, brominated n-butylpyridine (0.015g / ml) 0.15ml, NaOH (8mol / L) 0.15ml and H 2 O 10ml, vigorously stirred, the temperature was controlled at 20°C, and reacted for 1.5h; the reacted mixture was extracted with ethyl acetate, and then concentrated under reduced pressure; The peak area was measured at 251 nm of the characteristic peak of ketone, and the content of flavanone was calculated, and the yield was 73.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com