Compound having affinity with progeria dementia A beta plaques, preparation method and application thereof
A compound and a dementia technology, which are applied to compounds having affinity with Alzheimer's Aβ plaques, their preparation and application fields, and can solve the problems of harsh synthetic process conditions, low yield, and difficult synthesis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
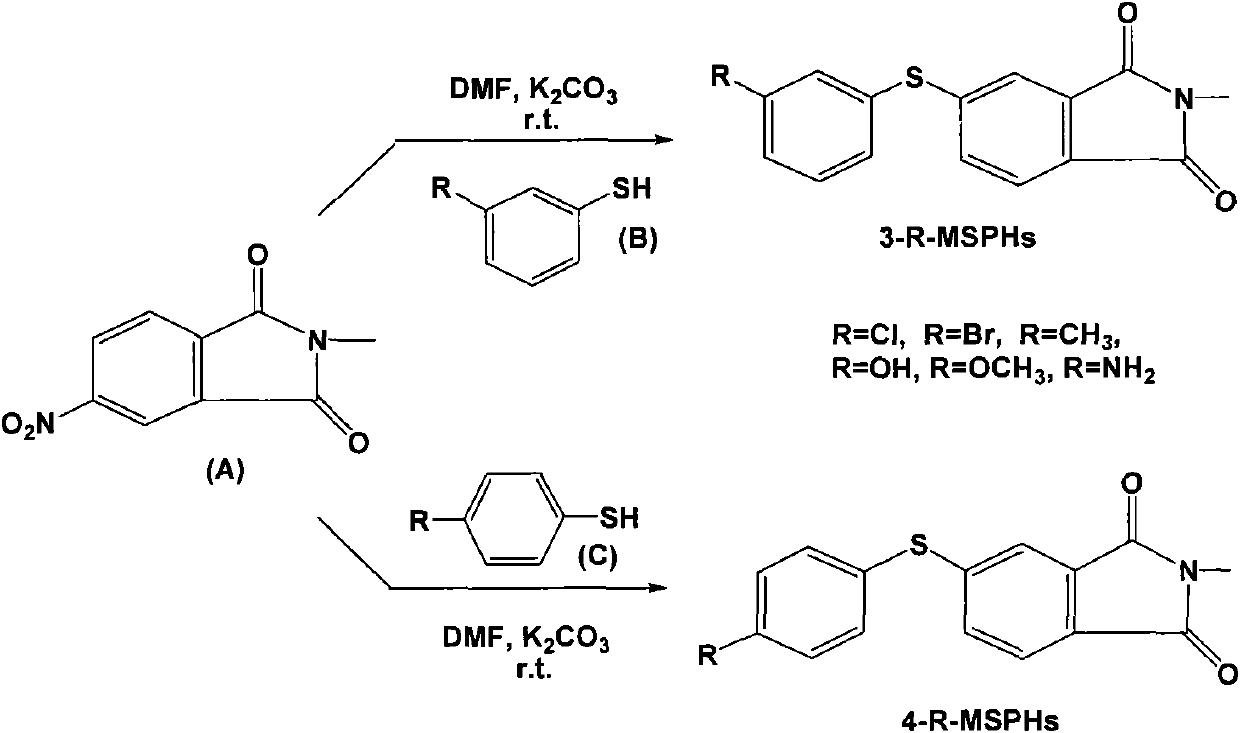
[0084] Example 1 Preparation of N-methyl-4-(3-chlorophenylthio)phthalimide (3-Cl-MSPH)
[0085] Dissolve 1.03 g of N-methyl-4-nitrophthalimide and 5 mmol of 3-chlorothiophenol in 15 mL of N, N-dimethylformamide (DMF), and add 6 mmol anhydrous K 2 CO 3 , stirring at room temperature (25° C.) for 12 hours to perform a coupling reaction;
[0086] Remove K by suction filtration under reduced pressure 2 CO 3 After that, the solvent N, N-dimethylformamide was distilled off under reduced pressure, the residue was dissolved in ethyl acetate, and then water was added for repeated extraction until the water phase was neutral;
[0087] with anhydrous MgSO 4 Dry the ethyl acetate phase, use a rotary evaporator to remove ethyl acetate, and recrystallize the residue with methanol to obtain off-white crystals of N-methyl-4-(3-chlorophenylthio)phthalimide (3- Cl-MSPH), the yield of 3-Cl-MSPH: 51.2%; m.p.135-136 ℃; IR (cm -1 ): 3450, 1714, 1701, 1604, 1438, 735, 680; 1 H NMR (500MHz, C...
Embodiment 2
[0089] Example 2 Preparation of N-methyl-4-(4-chlorophenylthio)phthalimide (4-Cl-MSPH)
[0090] In addition to using 4-chlorothiophenol and N-methyl-4-nitrophthalimide dissolved in dichloromethane, the coupling reaction was carried out under the action of catalyst anhydrous sodium carbonate to obtain off-white crystals Except N-methyl-4-(4-chlorophenylsulfanyl) phthalimide (4-Cl-MSPH), all the other are the same as Example 1, and the productive rate of 4-Cl-MSPH is 60.7% ; m.p. 136-136.5°C. IR (cm -1 ): 3444, 1715, 1706, 1650, 1607, 1435, 737, 501. 1 H NMR (500MHz, CDCl 3 , δppm): 7.69 (1H, d, J=7.8Hz), 7.52 (1H, s), 7.49 (5H, m), 3.15 (3H, s). HRMS: calcd for C 15 h 10 NO 2 SClNa, 326.0018; found: 326.0023; the structural formula is
Embodiment 3
[0091] Example 3 Preparation of N-methyl-4-(3-bromophenylthio)phthalimide (3-Br-MSPH)
[0092] In addition to using 3-bromothiophenol and N-methyl-4-nitrophthalimide dissolved in acetone, the coupling reaction was carried out to obtain light yellow granular crystal N-methyl-4-(3 -Bromophenylthio) phthalimide (3-Br-MSPH), the rest are the same as in Example 1, and the yield of 3-Br-MSPH is 49.1%; m.p.131.5-132°C; IR ( cm -1 ): 3451, 2359, 1715, 1700, 1601, 1436, 736, 676; 1 H NMR (500MHz, CDCl 3 , δppm): 7.67 (1H, d, J = 8.2Hz), 7.64 (1H, s), 7.54 (2H, m), 7.48 (1H, d, J = 7.8Hz), 7.42 (1H, d, J = 7.8Hz), 7.29(1H, d, J=7.9Hz), 3.16(3H, s); HRMS: calcd for C 15 h 10 NO 2 SBr, 347.9694; found: 347.9705;
[0093] The structural formula is
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com