Preparation method for biapenem condensation compound and crystalline solid thereof
A technology for crystals and compounds, which is applied to the preparation of biapenem condensate and the field of crystals, can solve problems such as unfavorable cost saving, and achieve the effect of facilitating solid-liquid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation method of biapenem condensate crystals 1
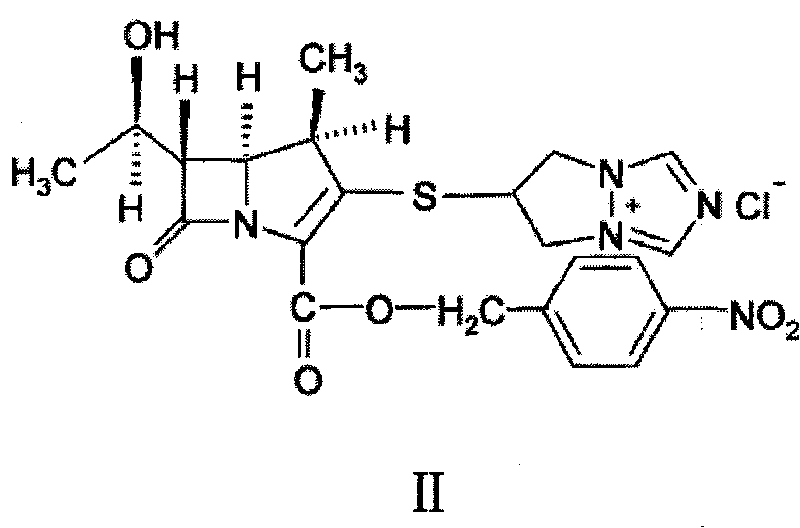
[0040] 1 kg of 6,7-dihydro-6-mercapto-5H-pyrazol[1,2-α][1,2,4]triazole ylide and (4R,5S,6S)-3-diphenyl Oxyphosphoryloxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo「3.2.0」hept-2-ene-2-carboxy Add 2.5kg of p-nitrobenzyl acid to 12L of acetonitrile, then add 5L of ethanol, stir, cool down to about 0°C, add diisopropylethylamine dropwise, react at the same temperature for 2 to 5 hours, filter, wash, After drying, 1.95 kg of light yellow biapenem condensate crystals were obtained, with an HPLC purity of 98.7%. The obtained alpenem condensate crystals were analyzed by powder X-ray diffraction, the results are shown in the attached figure 1 ,Table 1.
[0041] Table 1
[0042] d value
[0043] The obtained biapenem condensate crystals were analyzed using KBr pellets to obtain infrared absorption spectra at 3415, 3147, 2964, 1763, 1695, 1605, 1552, 1518, 1442, 1403, 1384, 1343, 1322, 1289, 1221, 1178, ...
Embodiment 2~5
[0045] Preparation method of biapenem condensate crystals 2-5
[0046] Referring to the preparation method of Example 1, the lower alcohol solvent ethanol was changed to methanol, n-propanol, isopropanol, and tert-butanol to obtain pale yellow biapenem condensate crystals. The experimental results are shown in Table 2.
[0047] Table 2 embodiment 2~5 experimental result
[0048] Example number
[0049] Powder X-ray diffraction analysis and KBr tablet infrared analysis of the obtained crystals of the condensate of biapenem showed that the crystal form of the crystals of the obtained condensate of biapenem was consistent with the crystal form of the crystals obtained in Example 1.
Embodiment 6
[0051] Preparation of biapenem
[0052]Add 1.8 kg of biapenem condensate crystals obtained by the method of the present invention into 10 L of tetrahydrofuran, 30 L of 0.3 mol / L phosphate buffer (pH5.6), stir and dissolve, add 0.3 kg of 20% palladium carbon, and control the pressure at 10 kg / L. m 2 , react at 40°C for 30 min, filter, wash the aqueous phase with 20 L of ethyl acetate × 2, add 40 L of acetone, stir at room temperature for 2 h, filter, wash the solid with acetone, and dry under reduced pressure to obtain 920 g of biapenem, HPLC purity 98.2% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com