Method for preparing lithium fluoride
A variety of lithium fluoride and lithium fluoride technologies, applied in the direction of lithium halide, etc., can solve the problems of lithium fluoride purity not meeting the requirements, poor fluidity, and high alkaline impurities, so as to avoid product quality and good fluidity , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
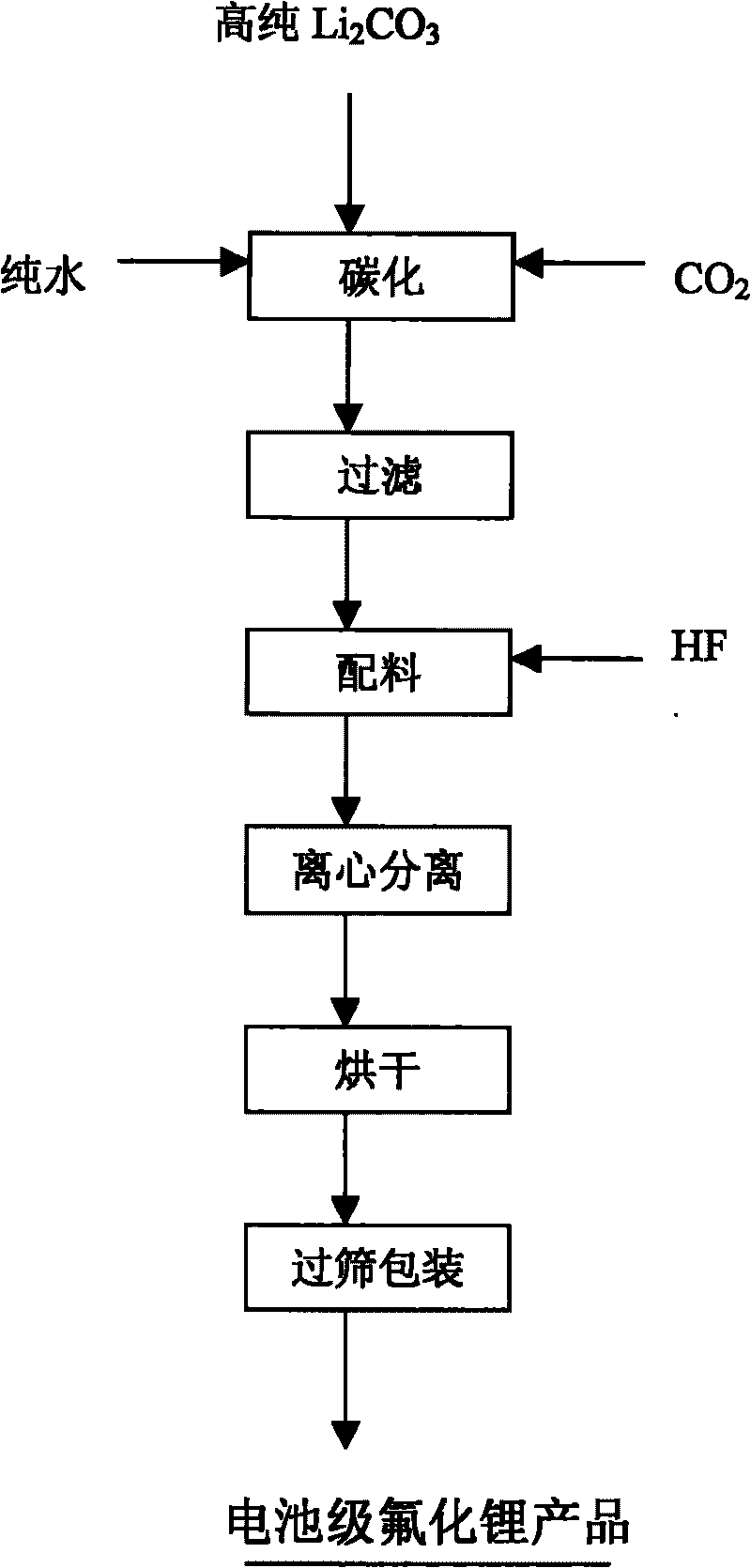
[0017] The reaction principle of producing lithium fluoride in this example is:
[0018] Li 2 CO 3 +H 2 O+CO 2 ==2LiHCO 3 (P CO2 =0.40~0.45MPa)
[0019] LiHCO 3 +HF=LiF↓+CO 2 ↑+H 2 o
[0020] Main production process:
[0021] 1. Carbonation reaction
[0022] Add 2.5m3 pure water into the carbonization kettle, then put in 75kg of high-purity lithium carbonate, and cover the hand hole tightly. Start stirring, turn on the circulating cooling water, then slowly open the carbon dioxide gas control valve, raise the pressure in the carbonization kettle to 0.4MPa for reaction, and continue to maintain this pressure for 3 hours to obtain lithium bicarbonate solution.
[0023] The specifications of the main raw materials used above are
[0024] 1. Pure water quality requirements (ppm)
[0025]
[0026] 2. Quality requirements for high-purity lithium carbonate (ppm)
[0027]
[0028] 3. Carbon dioxide: industrial grade, the content is not less than 99.9%.
[0029] 2...
Embodiment 2
[0055] Main production process:
[0056] 1. Carbonation reaction
[0057] Add 2m into the carbonization kettle 3 Pure water, then put in 100kg of high-purity lithium carbonate, and cover the hand hole tightly. Start stirring, turn on the circulating cooling water, then slowly open the carbon dioxide gas control valve, raise the pressure in the carbonization kettle to 0.45MPa for reaction, and continue to maintain this pressure for 2.5 hours to obtain lithium bicarbonate solution.
[0058] The purity of the main raw materials used above, pure water, high-purity lithium carbonate and carbon dioxide raw materials are all greater than 99.9%
[0059] 2. Filtering:
[0060] After the carbonization reaction is complete, the carbonized solution is filtered through a 5 μm sintered ceramic precision filter, and the filtrate is poured into the batching tank.
[0061] 3. Neutralization reaction:
[0062] Start the stirring of the lithium bicarbonate solution in the batching tank, tur...
Embodiment 3-5
[0072]
[0073] The lithium fluoride product that above-mentioned embodiment produces all can reach the requirement of the object of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com