Stable injection composite of 12 complex vitamins and preparation method thereof
A technology of complex vitamins and vitamins, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve problems that threaten the safety of clinical medication, and achieve the effect of low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
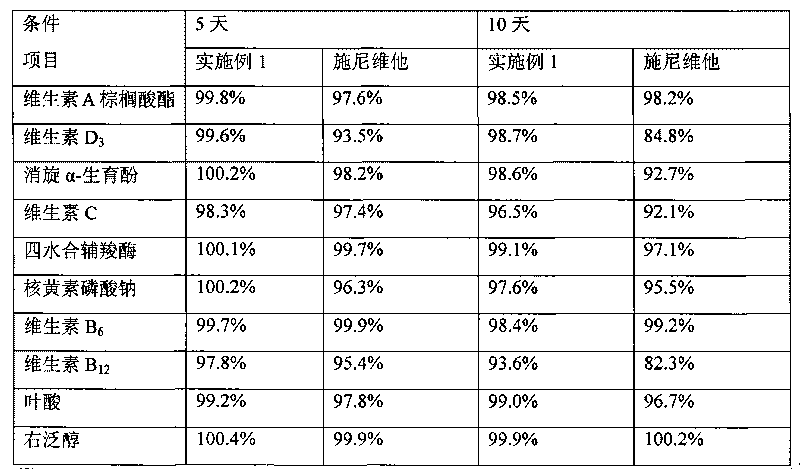
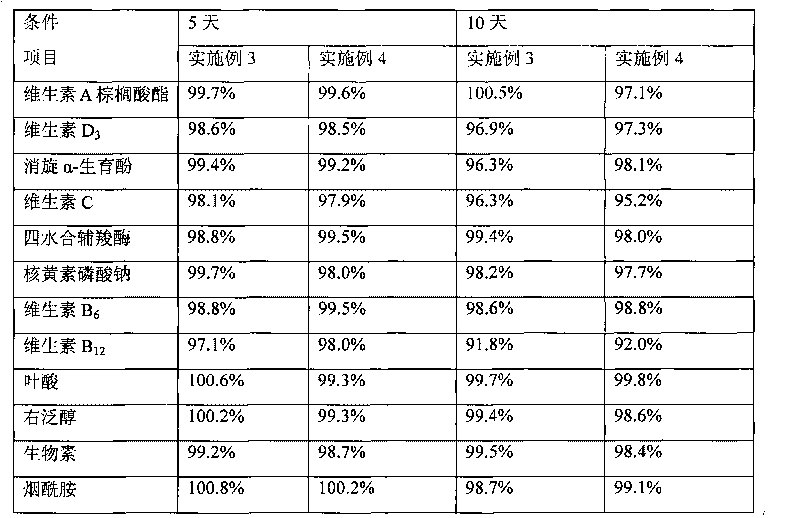
Image
Examples
Embodiment 1
[0196] prescription:
[0197] Raw materials Prescription
[0198] Vitamin A palmitate 3500000IU
[0199] Vitamin D 3 220000IU
[0200] Racemic alpha-tocopherol 10.2g
[0201] Vitamin C 125g
[0202] Cocarboxylase Tetrahydrate 5.8g
[0203] Riboflavin Sodium Phosphate 5.67g
[0204] Vitamin B 6 5.5g
[0205] Vitamin B 12 0.006g
[0206] Folic acid 0.414g
[0207] Dexpanthenol 16.15g
[0208] Biotin 0.069g
[0209] Niacinamide 46g
[0210] Polysorbate 80 200g
[0211] Glycine 550g
[0212] sodium hydroxide solution
[0213] Add water for injection to 5000ml
[0214]
[0215] Makes 1000 bottles
[0216] Preparation Process:
[0217] (1) In a light-proof operation room, weigh vitamin A palmitate, racemic α-tocopherol, and vitamin D 3 , added to polysorbate 80, charged with nitrogen, stirred to dissolve, added with water for injection, stirred, and pla...
Embodiment 2
[0223] prescription:
[0224] Raw materials Prescription
[0225] Vitamin A palmitate 3500000IU
[0226] Vitamin D3 220000IU
[0227] Vitamin E 11.2g
[0228] Vitamin C 125g
[0229] Cocarboxylase Tetrahydrate 5.8g
[0230] Riboflavin Sodium Phosphate 5.67g
[0231] Vitamin B 6 5.5g
[0232] Vitamin B 12 0.006g
[0233] Folic acid 0.414g
[0234] Dexpanthenol 16.15g
[0235] Biotin 0.069g
[0236] Niacinamide 46g
[0237] Polysorbate 80 90g
[0238] Mannitol 460g
[0239] sodium hydroxide solution
[0240] Add water for injection to 5000ml
[0241]
[0242] Makes 1000 bottles
[0243] Preparation process: except that vitamin E is replaced with racemic α-tocopherol and mannitol is replaced with glycine, other preparations are carried out as in Example 1, and the product is obtained.
Embodiment 3
[0245] prescription:
[0246] Raw materials Prescription
[0247] Vitamin A palmitate 3500000IU
[0248] Vitamin D 3 220000IU
[0249] Racemic alpha-tocopherol 10.2g
[0250] Vitamin C 125g
[0251] Cocarboxylase Tetrahydrate 5.8g
[0252] Riboflavin Sodium Phosphate 5.67g
[0253] Vitamin B 6 5.5g
[0254] Vitamin B 12 0.006g
[0255] Folic acid 0.414g
[0256] Dexpanthenol 16.15g
[0257] Biotin 0.069g
[0258] Niacinamide 46g
[0259] Polysorbate 80 200g
[0260] Mannitol 480g
[0261] sodium hydroxide solution
[0262] Add water for injection to 5000ml
[0263]
[0264] Makes 1000 bottles
[0265] Preparation process: except that mannitol is replaced by glycine, other preparations are carried out as in Example 1, and the product is obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com