Protease, preparation method of same, as well as application and pharmaceutical formulation thereof
A protease and dosage form technology, applied in the fields of medicine, insects, and enzymology, can solve the problems of short half-life, strong bleeding side effects, and difficult to control the dosage of drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment one: the separation and preparation of protease
[0041] Tussah silkworm (Antheraea pernyi) belongs to Lepidoptera (Lepidoptera) Saturniidae, is a kind of economic insect mainly raised in the wild in northern my country. The experimental material used in the present invention is the live tussah silkworm chrysalis sold in the market. Take tussah silkworm chrysalis, incubate at 25°C until the moth emerges, and collect the exudate from the mandibular gland. Take the spit out, put it in a centrifuge tube, adjust the pH to 6.0 with buffer solution 0.02mol / L HAC-NaAC (pH6.0), centrifuge at 12000r / min for 10min, and take the supernatant for later use (if the sample is less than 2ml, use buffer solution 0.02 mol / LHAC-NaAC, pH6.0 make up to 2ml). The cation exchange column (HiTrap TM SP-FF), and then equilibrate with Binding Buffer (0.02mol / L HAC-NaAC, pH 6.0) of 5 times the column volume at a flow rate of 2ml / min. Take 2ml of the sample for loading, and then use ...
Embodiment 2
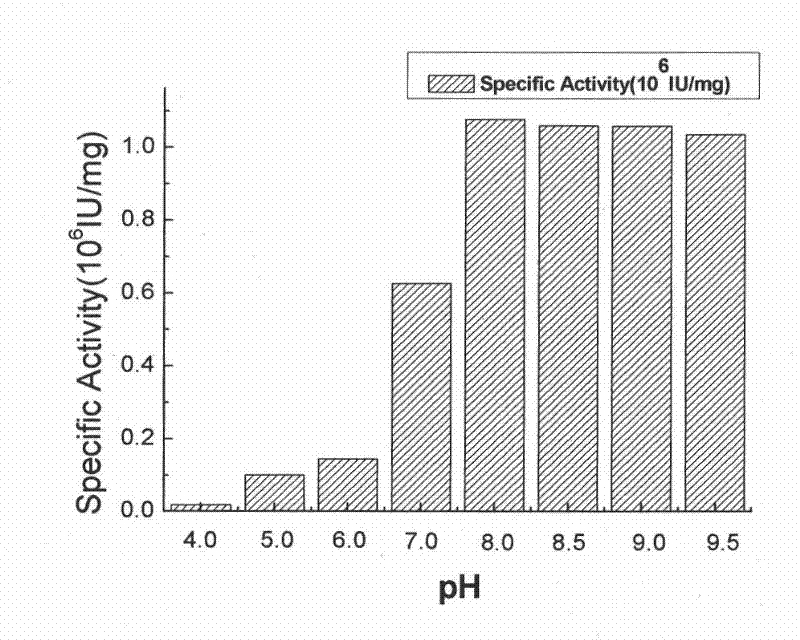
[0042] Example 2: Analysis of plasmin activity of protease and determination of enzymatic properties
[0043] 1. Degradation of fibrinogen and determination of plasmin activity
[0044] (1) Degradation of fibrinogen
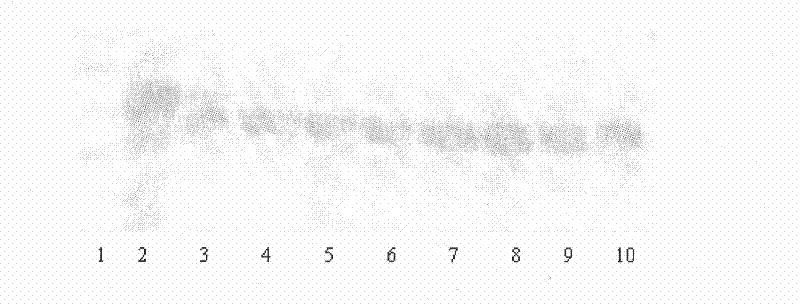
[0045] Take 0.2% human fibrinogen (0.1mol / L Tris-HCL, pH 8.0) solution and 10 microliters of purified protease, mix and incubate at 37°C for 0, 0.5, 1, 2, 3, 4, 8, At 16 and 24 hours, denaturant (10M urea, 4% SDS, 4% 2-mercaptoethanol) was added to stop the reaction, and SDS-PAGE was used for determination. Such as figure 2 , showed that tussah protease could effectively hydrolyze the Aα-chain and Bβ-chain of fibrinogen, but the hydrolysis activity on the γ-chain was weak. The results calculated by Bandscan software are as follows: image 3 As shown in .4, the tussah protease firstly hydrolyzes the Aα-chain of fibrinogen, and can hydrolyze more than 90% within 2 hours; it takes 4 hours to reach 90% for the hydrolysis of the Bβ-chain, and it takes 4 hours for...
Embodiment 3
[0058] Example 3: Analysis of Thrombolytic Effect of Protease in Vitro
[0059] 1. Detection of thrombolytic effect
[0060] Kunming rats were anesthetized with ether, and blood was collected by cardiac puncture. After the blood coagulates, it is cut into uniform small pieces and weighed. The blood clot was added to 0.5 mL tussah protease (0.02 mmol / L NaAC-HAC) solution, and 0.02 mmol / L NaAC-HAC was used as a control, and placed in a constant temperature water bath at 37°C. Every 10 minutes, slightly oscillate, observe, record and take pictures regularly. As a result, it was found that the solution in the upper part of the control was clearer, and the thrombus was not obviously dissolved. The solution contained a very small amount of red blood cells. In the tussah protease group, the solution in the upper part was turbid, and the thrombus was dissolved obviously. The solution contained a large amount of cells released from the thrombus. See the appendix Figure 5 .
[0061...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com