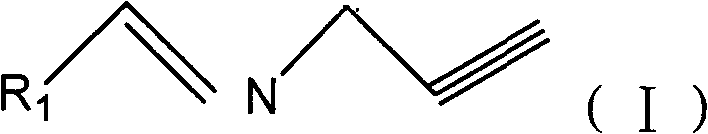
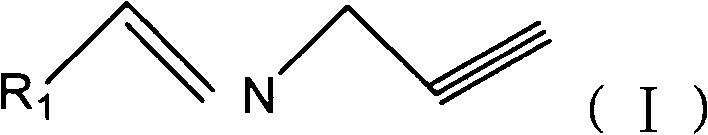
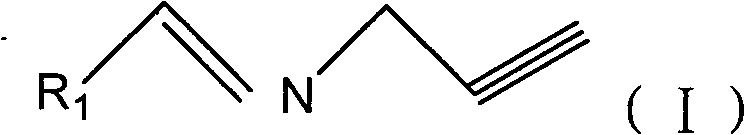
Propargyl imine compounds and use thereof in preparation of MAO inhibitor
A propargyl imine compound technology, applied in the field of new propargyl imine compounds, can solve the problems of limited application, and achieve the effect of simple equipment, simple operation process and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of (3,5-dimethyl-2-propynyl imine)-1H-pyrrole (compound 1)
[0032] (3,5-Dimethyl-1H-pyrrole)-2-carbaldehyde 0.25g (2mmol) was dissolved in dry THF (100ml), after the dissolution was complete, 4.0ml (60mmol) of propargylamine, anhydrous magnesium sulfate 5.0 g (40mmol), stirred at room temperature for 48h. Filter the filtrate to remove the solvent with a rotary evaporator, extract the residue with dichloromethane, wash with saturated brine (100ml) and saturated sodium bicarbonate solution (100ml), separate the organic layer, dry over anhydrous magnesium sulfate, filter, and evaporate to dryness , to obtain the crude product, column chromatography (silica gel is the stationary phase, the developer is ethyl acetate / petroleum ether, gradient concentration, the product is obtained when the volume ratio of ethyl acetate / petroleum ether is 1:1, the same below), and a yellow solid 0.278 g, yield 87%.
[0033]
Embodiment 2
[0034] Embodiment 2: the synthesis of diphenylpropynyl imine (compound 2)
[0035] Dissolve 0.36g (2mmol) of benzophenone and 4ml of propargylamine in dry 15ml of dichloromethane, cool to -20°C, add 3ml of titanium tetrachloride with a syringe, and react at room temperature for 3 days. After the reaction is complete, filter , adding dichloromethane to the filtrate for extraction, washing with saturated brine and sodium bicarbonate solution, separating the organic layer, drying over anhydrous magnesium sulfate, filtering, and evaporating to dryness to obtain the crude product, which was purified by column chromatography to obtain 0.4166g of a yellow liquid, Yield 96%.
[0036]
Embodiment 3
[0037] Example 3: Synthesis of (5,6-dimethoxy)phenylpropynyl imine (compound 18)
[0038] Dissolve 0.332g (2mmol) of 5,6-dimethoxybenzaldehyde in 100ml of benzene, add 2ml of propargylamine, 0.5g of anhydrous magnesium sulfate, reflux at 80°C for 18h, filter, and remove the solvent from the filtrate with a rotary evaporator , the residue was extracted with dichloromethane, washed with saturated brine (100ml) and saturated sodium bicarbonate solution (100ml), separated the organic layer, dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness to obtain crude product, column chromatography, to obtain Reddish-brown liquid 0.37g, yield 92%.
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com