Rabbit monoclonal antibody based ciprofloxacin residue analysis enzyme-linked immune adsorption kit
An enzyme-linked immunosorbent, monoclonal antibody technology, applied in the field of kits, can solve problems such as no reports, and achieve the effect of reducing detection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
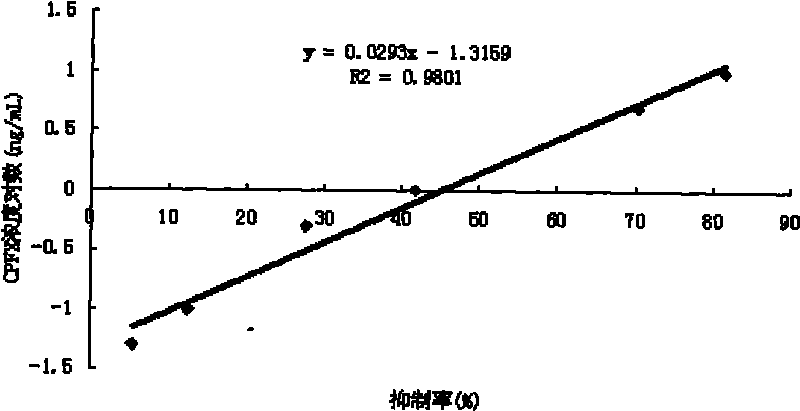
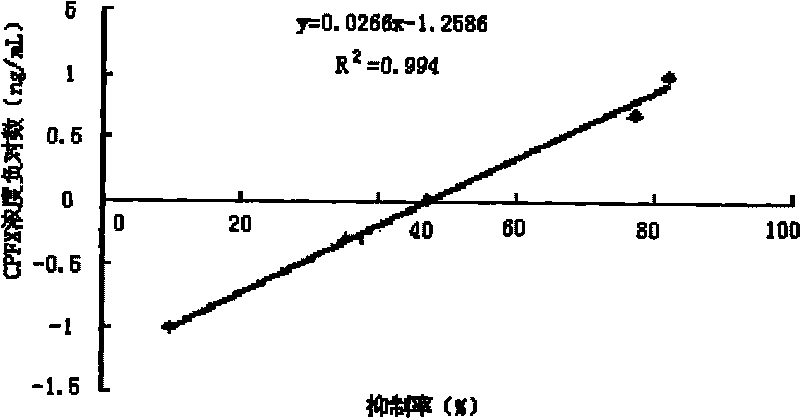
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of Ciprofloxacin Immunization Antigen and Coating Antigen
[0027] The specific operation steps are as follows: add 5.76mg NHS, 20.64mg EDC, 3.86mg CPFX to 0.23ml DMF, incubate at room temperature, shading, for 24h (solution I); add 26.4mg BSA to 2.3ml 0.01MpH7.4 PBS (solution II). Then, the incubated solution I was added dropwise to solution II, shaken while adding, and stirred magnetically for 3 hours at room temperature. The reaction mixture was transferred into a dialysis bag and dialyzed against 0.01M pH7.4 PBS to remove free CPFX and other small molecular substances. The coating antigen OVA-CPFX was prepared in the same way and used to coat the microtiter plate.
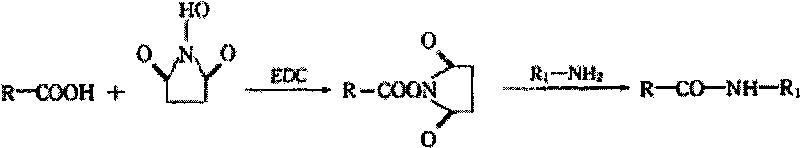
[0028] The coupling principle of ciprofloxacin and carrier protein is as follows:
[0029]
[0030] Note: R-COOH is ciprofloxacin: For the NHS: R-CO-NH-R 1 for coupling products
Embodiment 2
[0031] Example 2: Production of Ciprofloxacin Rabbit Monoclonal Antibody
[0032] 1. Immunization of rabbits
[0033] For primary immunization, mix 500 μg of immune antigen with an equal amount of Freund’s complete adjuvant, and after complete emulsification, inject white rabbits (1 mL / only) subcutaneously at 5 points on the back; three weeks later, use 200 μg of immune antigen with Freund’s incomplete adjuvant After mixing, carry out the second immunization in the same way; after that, immunize once every two weeks. After a total of 3 to 7 immunizations, when the serum titer reaches 64,000 or more, inject 500 μg of immune antigen into the ear vein, and collect whole blood from the carotid artery 4 days later. , take the spleen aseptically and prepare for cell fusion.
[0034] 2. Preparation of rabbit monoclonal antibody
[0035]Rabbit spleen cells were fused with 240E in the logarithmic growth phase, with 50% PEG as the fusion agent and HAT as the selective medium, and spre...
Embodiment 3
[0036] Embodiment 3: the coating of microtiter plate
[0037] Ciprofloxacin-coated antigen was diluted to 4 μg / mL with pH9.6, 0.05mol / L carbonate buffer (containing 2.93g sodium bicarbonate and 1.59g sodium carbonate, dissolved in double distilled water or ultrapure water 1L) , add 100 μL to each well of the ELISA plate, coat at 4°C for 12 hours or 37°C for 2 hours, pour off the coating liquid, wash with PBST for 3 to 5 times, pat dry, then add 200 μL of 5% glycine to each well, Seal at 37°C for 1 hour, wash 3 to 5 times after taking it out, dry and seal the film for storage.
PUM
| Property | Measurement | Unit |
|---|---|---|
| reaction rate constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com