Chimeric polypeptide with dual-targeting function and applications thereof
A chimeric polypeptide and dual-targeting technology, which is applied in the preparation of protein-based polypeptides and its application field, to achieve the effects of increasing expression, strong pertinence, and reducing the number of medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
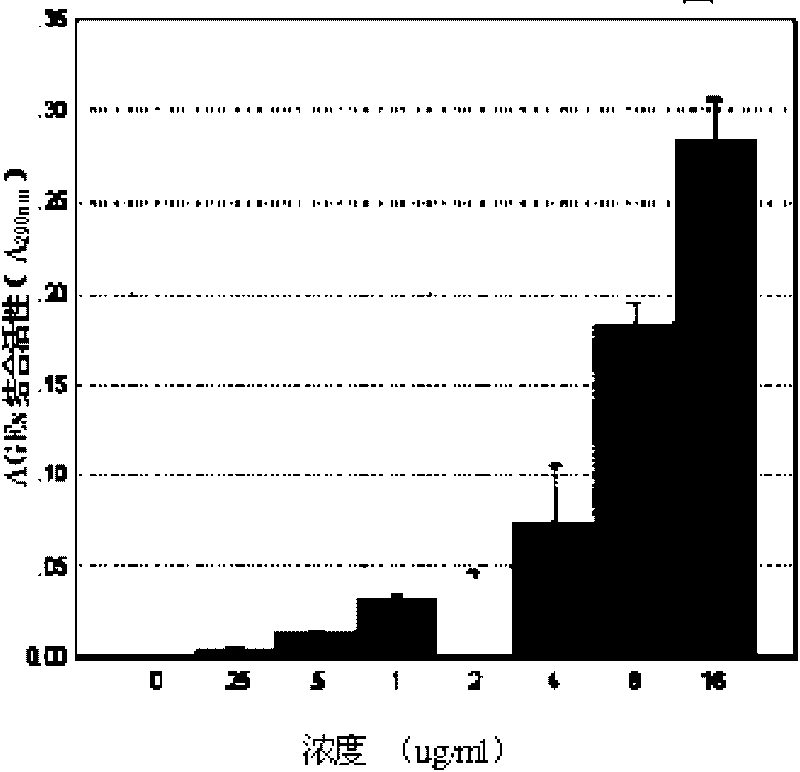
Image
Examples
Embodiment 1
[0029] 1. Preparation of chimeric polypeptide of the present invention
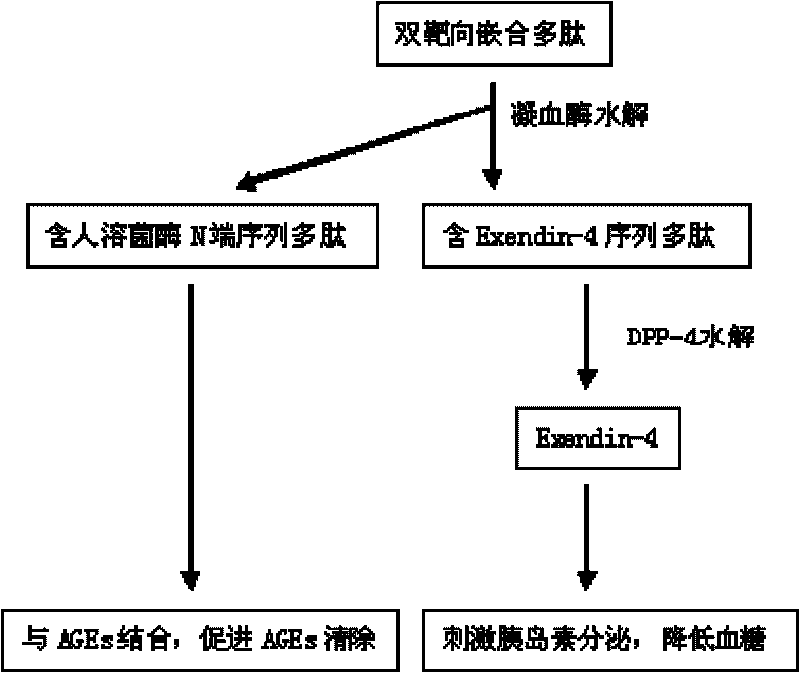
[0030] according to figure 1 According to the arrangement design, a chimeric peptide sequence composed of 80 amino acids at the N-terminal of lysozyme, 2 amino acid linking sequences and Exendin-4 was generated by genetic engineering technology, the sequence is as follows:
[0031] MKALIVLGLVLLSVTVQGKVFERCELARTLKRLGMDGYRGISLANWMCLAKWESGYNT
[0032] RATNYNAGDRSTDYGIFQINSRGPHGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS (SEQ ID No: 3)
[0033] The underline is the connection sequence, and P in the connection sequence can also be A, S, V, L.
[0034] The specific steps for preparing the above sequence are as follows:
[0035] 1. Obtain the DNA sequence of 80 amino acids at the N-terminal of human lysozyme and the cleavage sites of thrombin and dipeptidase by using PCR method or chemical synthesis method;
[0036] 2. Obtain the linking sequence containing thrombin and dipeptidase cleavage site and the DNA sequen...
Embodiment 2
[0048] according to figure 1 According to the arrangement design, a chimeric polypeptide consisting of 60 amino acids at the N-terminal of lysozyme, 6 amino acid linking sequences and Exendin-4 was synthesized by chemical synthesis, and then purified and prepared according to conventional methods. Peptide pharmaceutical ingredients.
[0049] The sequence of the chimeric polypeptide in this example is:
[0050] MKALIVLGLVLLSVTVQGKVFERCELARTLKRLGMDGYRGISLANWMCLAKWESGYNT
[0051] RALVPRGPHGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS (SEQ ID No: 4)
[0052] The underline is the connection sequence, L and V in the connection sequence can also be A, I, M, F, P, W, etc., and the P in the last position of G in the connection sequence can also be A, S, V, L.
Embodiment 3
[0054] according to figure 1 According to the arrangement design, a chimeric polypeptide composed of 40 amino acids at the N-terminal of lysozyme, 12 amino acid linking sequences, and Exendin-4 was synthesized by chemical synthesis, and then purified and prepared according to conventional methods for this chimeric polypeptide drug suitable for human Element.
[0055] The sequence of the chimeric polypeptide in this example is:
[0056] MKALIVLGLVLLSVTVQGKVFERCELARTLKRLGMDGYRGLVPRGPLVPRGPHGEGTFTSDLSKQMEEEAVRLFIEWLKNGGPSSGAPPPS (SEQ ID No: 5)
[0057] The underline is the connection sequence, L and V in the connection sequence can also be A, I, M, F, P, W, etc., and the P in the last position of G in the connection sequence can also be A, S, V, L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com