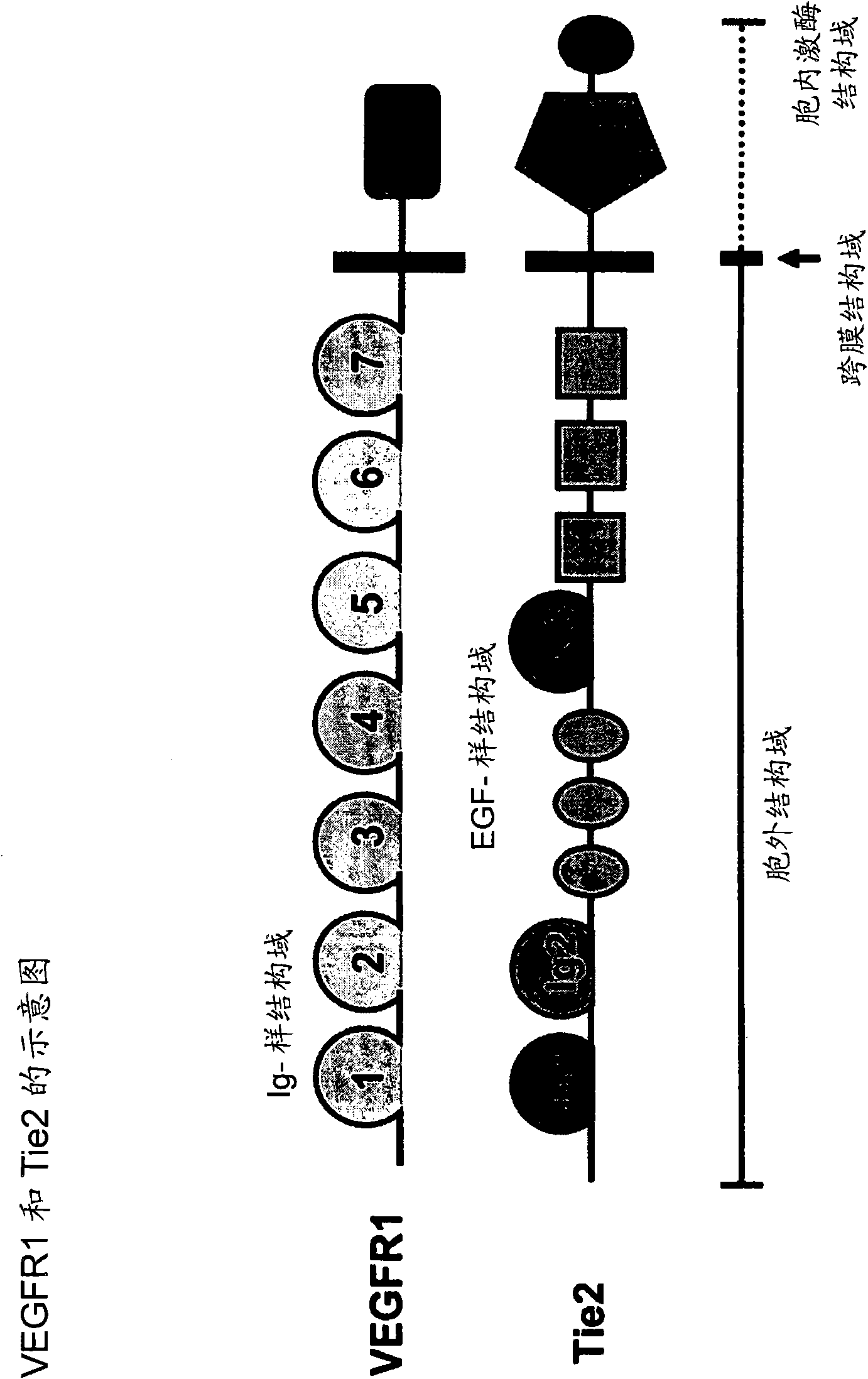
Fusion proteins binding to growth factors
A fusion polypeptide and simultaneous combination technology, which is applied in the direction of growth factors/growth regulators, fusion polypeptides, peptide/protein components, etc., can solve problems such as low pharmacokinetic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0166] Embodiment 1-experimental scheme
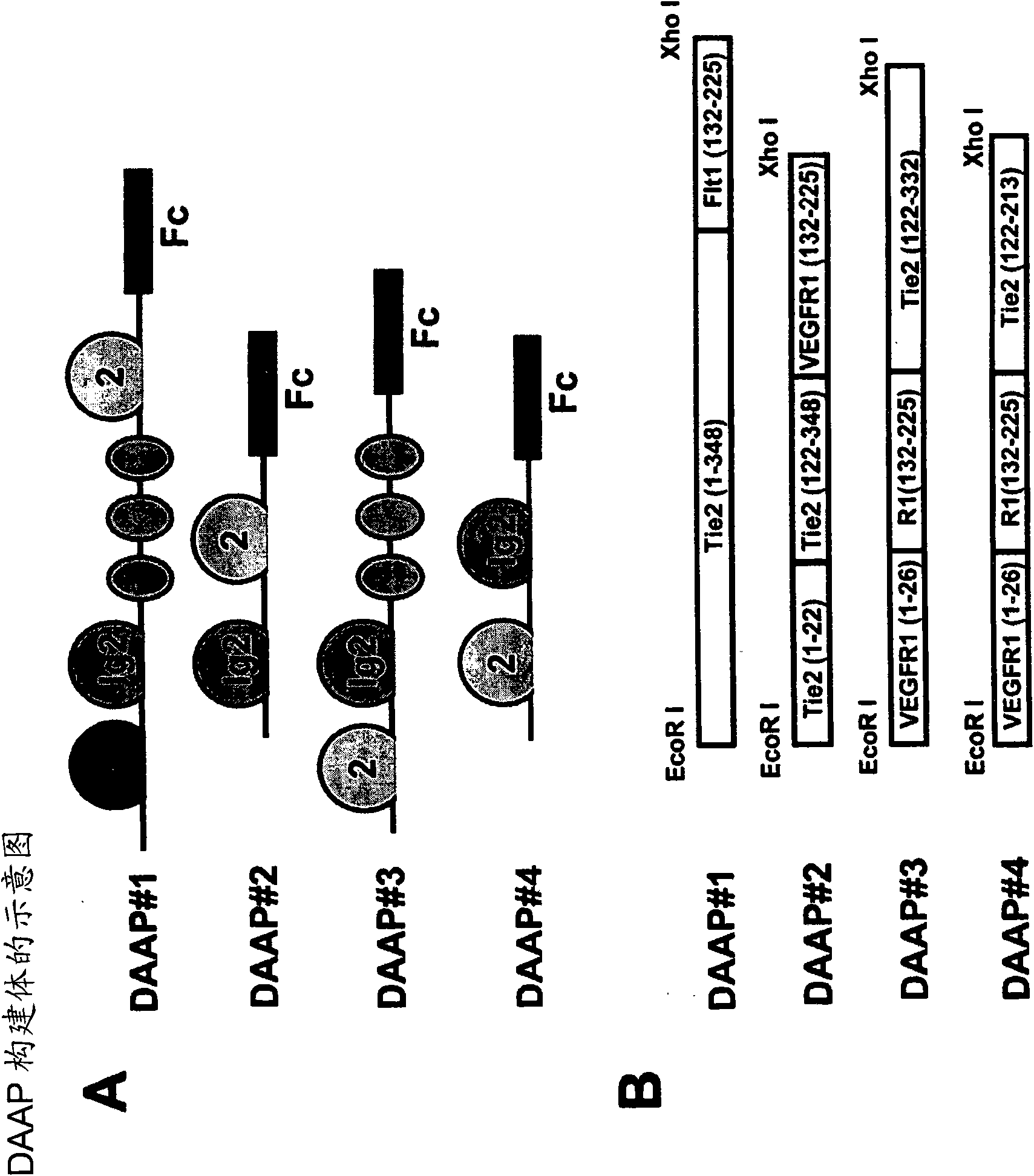
[0167] The code consists of the Ig-like domain 1 of human Tie2, the Ig-like domain 2 of human Tie2, the three EGF-like domains of human Tie2, the Ig-like domain of human VEGFR1 and the Fc domain of human IgG Four different assembled fusion proteins (DAAP#1, DAAP#2, DAAP#3 and DAAP#4) ( image 3 A and 3B) gene constructs were cloned into the pCMV-dhfr vector (Hwang SJ, et al., Protein Express Purif.2005; 39:175-183) ( Figure 4 ).
[0168] Since only DAAP#1 exhibited simultaneous binding to VEGF-A and Ang2, seven modified forms (DAAP#11, DAAP#12, DAAP# 13, DAAP #13, DAAP #14, DAAP #15, DAAP #16 and DAAP #17) ( Figure 5 with Image 6 ).
[0169] All DAAP recombinant proteins were obtained by transient expression in HEK293 cells (American Culture Collection of Type Microorganisms, Manassas, VA) using Effectene liposome transfection according to the manufacturer's instructions (Qiagen, Inc., Hilden, Germany) . Supernatants were col...
Embodiment 2
[0173] ELISA analysis showed that DAAP#1, DAAP#14, DAAP#16 and DAAP#17 could bind VEGF-A and Ang2( Figure 10 ). DAAP#2, DAAP#3, DAAP#4, DAAP#11 and Fc cannot bind to VEGF-A and Ang2 ( Figure 10 ). DAAP#12 and VEGF-trap can preferentially and selectively bind to VEGF-A, while DAAP#13 and Tie2-Fc can preferentially and selectively bind to VEGF-A ( Figure 10 ). Since DAAP#1 and DAAP#14 bind VEGF-A and / or Ang2 comparable to VEGF-trap or Tie2-Fc, DAAP#1 and DAAP#14 may be the strongest candidates for further development as therapeutic proteins candidate.
[0174] In order to verify whether the DAAP recombinant protein can simultaneously bind to VEGF and Ang2, 100 ng of each DAAP recombinant protein (DAAP#1, DAAP#12, DAAP#13, DAAP#14, DAAP#15, DAAP#16 and DAAP#17 ), with serial amounts (0 ng, 10 ng, 100 ng, 1,000 ng) of Ang2 or VEGF in 100 μl of blocking solution at 37 °C before they were added to the plates coated with VEGF-A or Ang2 used in the above ELISA -A pre-incubate...
Embodiment 3
[0177] Based on the above findings, DAAP#1 was further investigated for use as a therapeutic protein. Recombinant Chinese hamster ovary (rCHO) cells expressing DAAP#1 (CHO-DAAP#1) were established according to the method described previously (Hwang SJ, et al., Protein Express Purif. 2005; 39:175-183). Briefly, CHO-DAAP#1 cells were transfected into dhfr-deficient CHO cells (CRL-9096, American Culture Collection of Type Microorganisms) by transfecting a vector containing dihydrofolate reductase (dhfr) and a DAAP#1 gene construct. , Manassas, Virginia). This is followed by dhfr / methhotrexate (MTX)-mediated gene amplification. Stable rCHO cells secreting DAAP#1 were selected using sequentially expanded concentrations of MTX (0.02-1.0 [mu]M, Sigma-Aldrich). For recombinant DAAP#1 protein production, CHO-DAAP#1 cells were incubated at 37°C in a humidified 5% CO 2 In an incubator, on an orbital shaker (Vision, Bucheon, Korea) at 110 rpm, 2 × 10 5 cells / mL were inoculated. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com