Compositions comprising c-13 alkoxyether macrolide compounds and phenylpyrazole compounds
A compound, alkyl alkoxy technology, applied in the field of composition of ivermectin derivatives, can solve problems such as sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0655] Embodiment 1: the synthetic process of the MEM derivative of preparation ivermectin
[0656] The MEM derivative of ivermectin has the following formula:
[0657]
[0658] where R 16 =-CH 2 CH 3 When it is B1a type, R 15 =-CH 3 When the B1b type.
[0659] In order to synthesize the MEM derivative of ivermectin, the disaccharide group at the C-13 position of ivermectin is partially cleaved by acid solvolysis using 1% acid solution, resulting in cleavage into ivermectin aglycon. The -OH groups at C-5, C-7 and C-13 were then protected with tert-butyldimethylchlorosilane (TBDMS) followed by trimethylchlorosilyl (TMS) to obtain 7,13-Di-O-TMS-5-O-TBDMS ivermectin aglycone.
[0660] The 7,13-di-O-TMS-5-O-TBDMS ivermectin aglycon was then selectively deprotected using dichloroacetic acid to obtain the 7-O-TMS-5-O-TBDMS ivermectin glycoside Yuan. This aglycon was then alkylated with 2-methoxyethoxymethyl chloride (MEM chloride), followed by purification by crystalliza...
Embodiment 2
[0663] Embodiment 2: the anti-flea effect of the MEM derivative of ivermectin
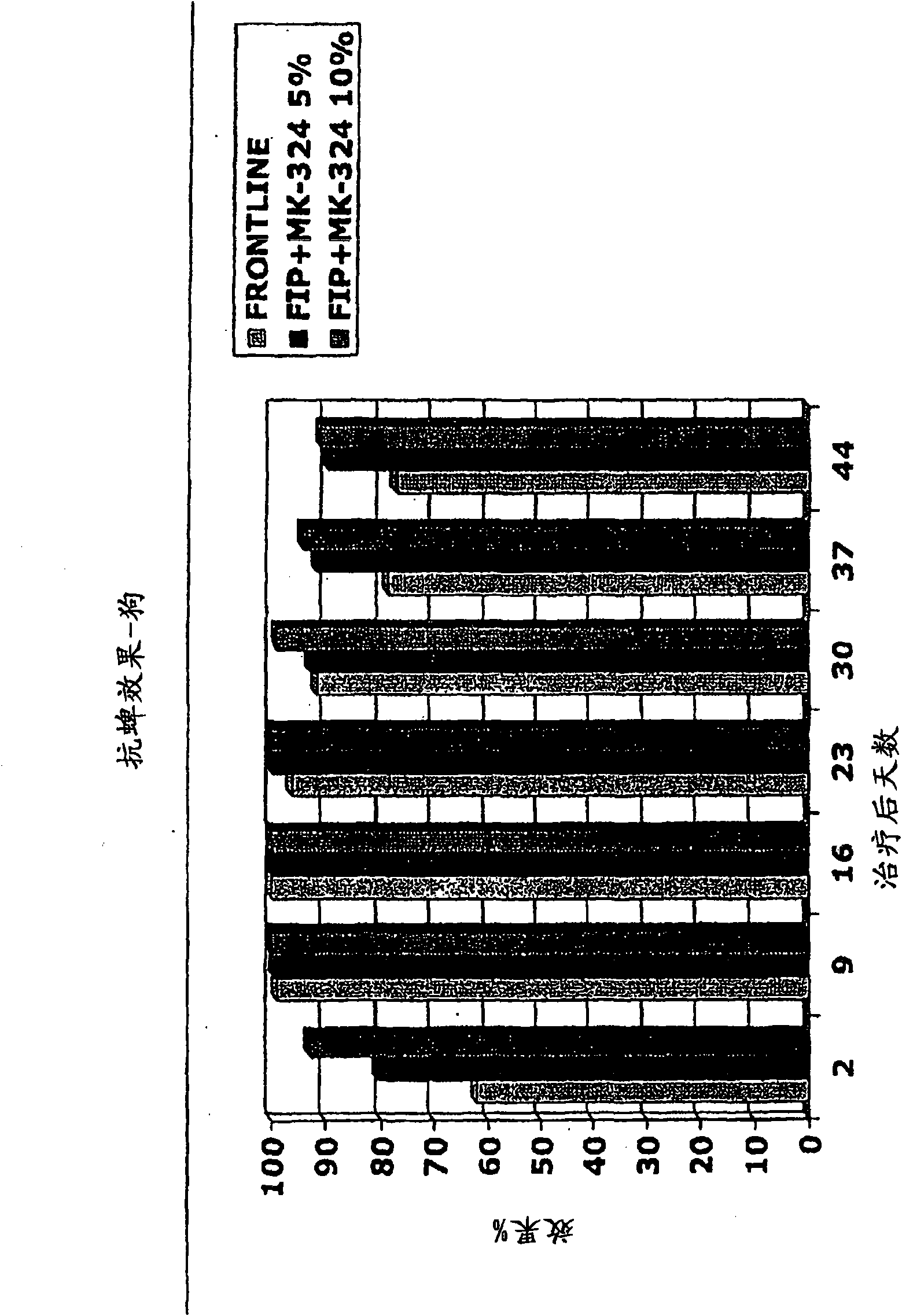
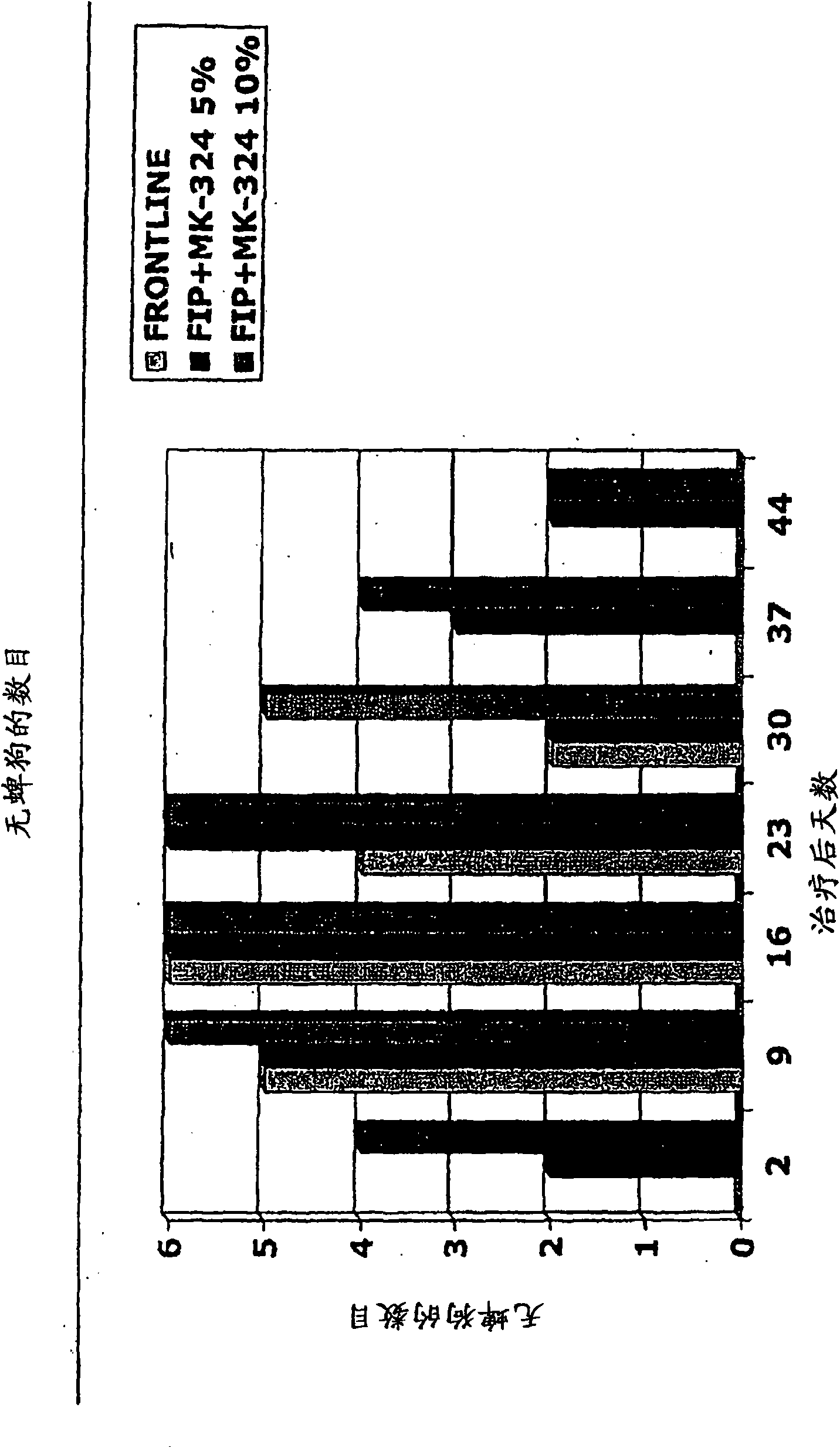
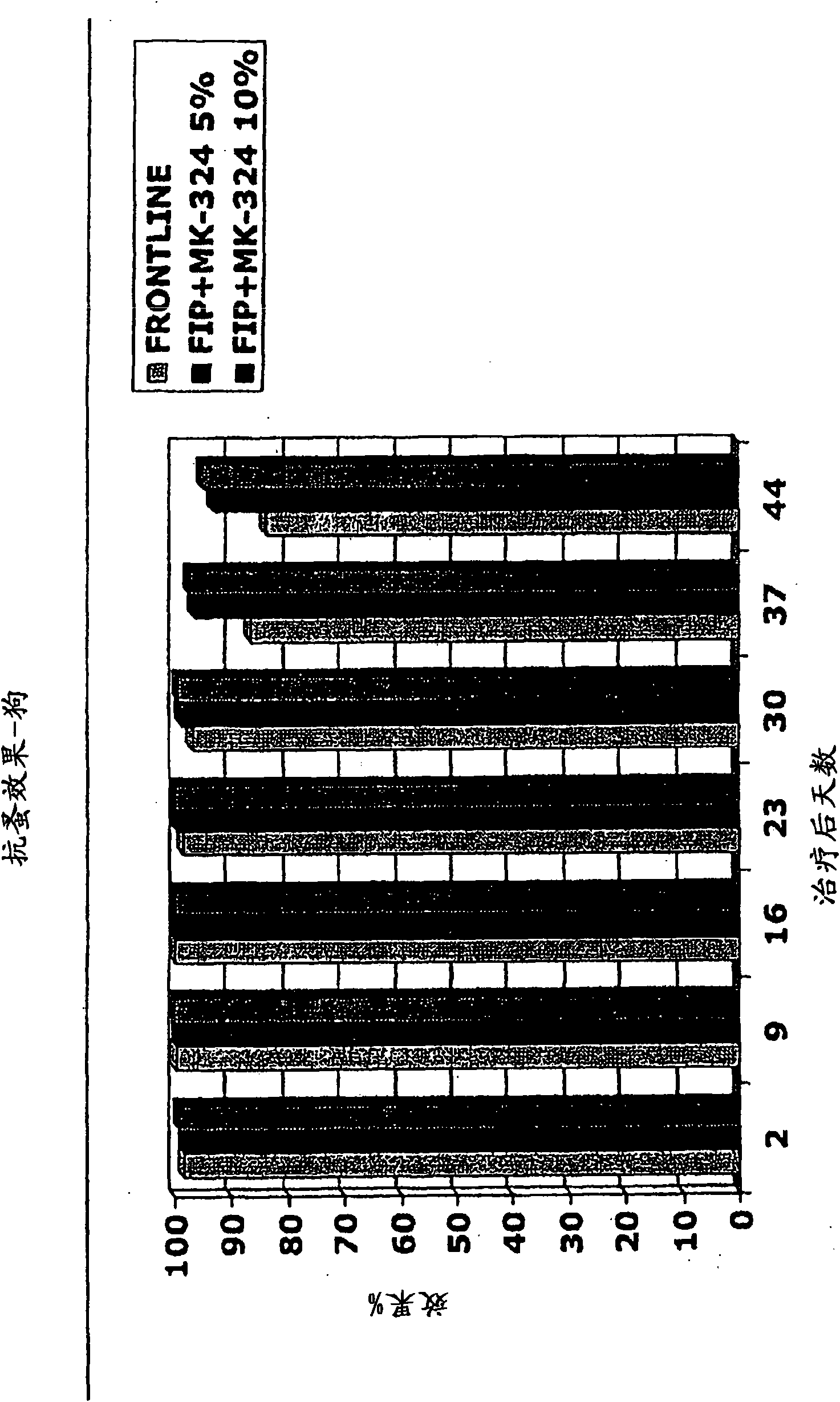
[0664] Two ivermectin derivatives (formula (III) and formula (IV)) were tested for their antiflea effect on male dogs. The efficacy of the compounds of formula (III) and (IV) against fleas was shown using 20 female dogs and 16 male dogs of different breeds with a body weight ranging from 9.3 to 21.6 kg. Dogs were assigned to treatment by limiting randomization based on pre-treatment (day 0) flea numbers. Treatment was vehicle control, 1.5% or 2.5% solution of compound of formula (III) applied topically at 1 ml / kg to provide doses of 15 or 25 mg / kg, 1% or 1.5% solution of compound of formula IV applied topically at 1 ml / kg to provide Doses of 10 or 15 mg / kg. Dogs are single-accommodated in cages or run wild indoors. On days -1, 13, 20 and 27 each dog was infected with 100 fleas (Ctentocephalus felis). On days 0, 1, 2, 3, 14, 15, 16, 21, 22, 23, 28, 29, 30, and 31, flea counts were performed by h...
Embodiment 3
[0666] Example 3: Effect of MEM derivatives of ivermectin against sarcoptic mangae
[0667] Two ivermectin derivatives (formula (III) and formula (IV)) were tested for their anti-scabies effect on male dogs. Twenty-three hybrid dogs and one Heeler (Australian Shepherd), weighing 2.9 to 20.0 kg, with an estimated age of 3 months to 7 years, and naturally infected with sarcoidosis canis, were topically applied according to the multi-point application system, and the These dogs were used to compare the effects of compounds of formula (III) and formula (IV). There are 12 male dogs and 12 female dogs.
[0668] The dogs were placed individually in the kennel. Replication groups of four dogs were formed based on animal availability and mite count; in the repetition groups, dogs were randomly assigned to either vehicle treatment (L-930,870) or to receive the following: Formula (III) at 15 mg / kg body weight The groups of compounds were either 10 mg / kg of the compound of formula IV o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com