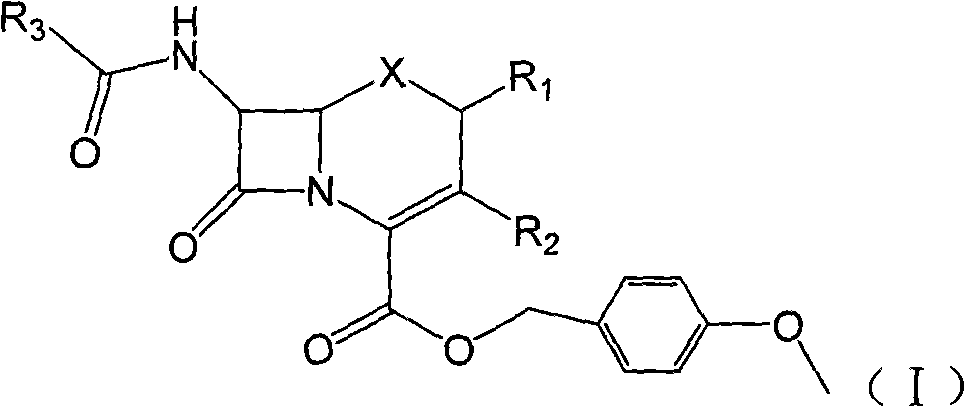
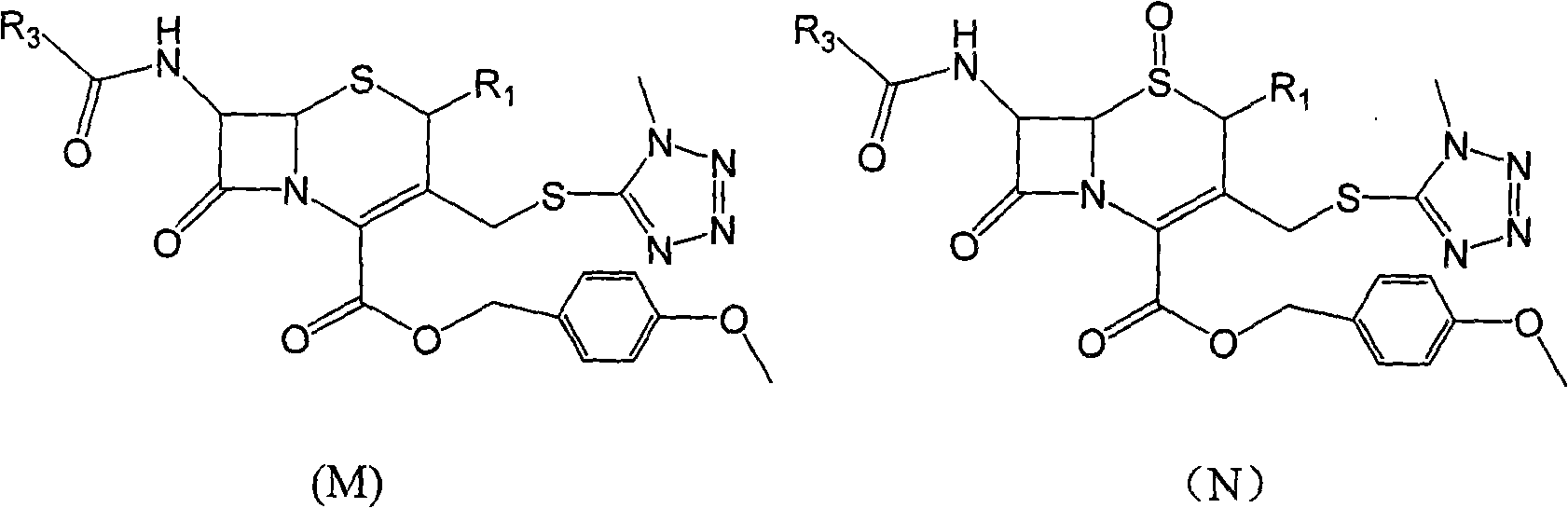
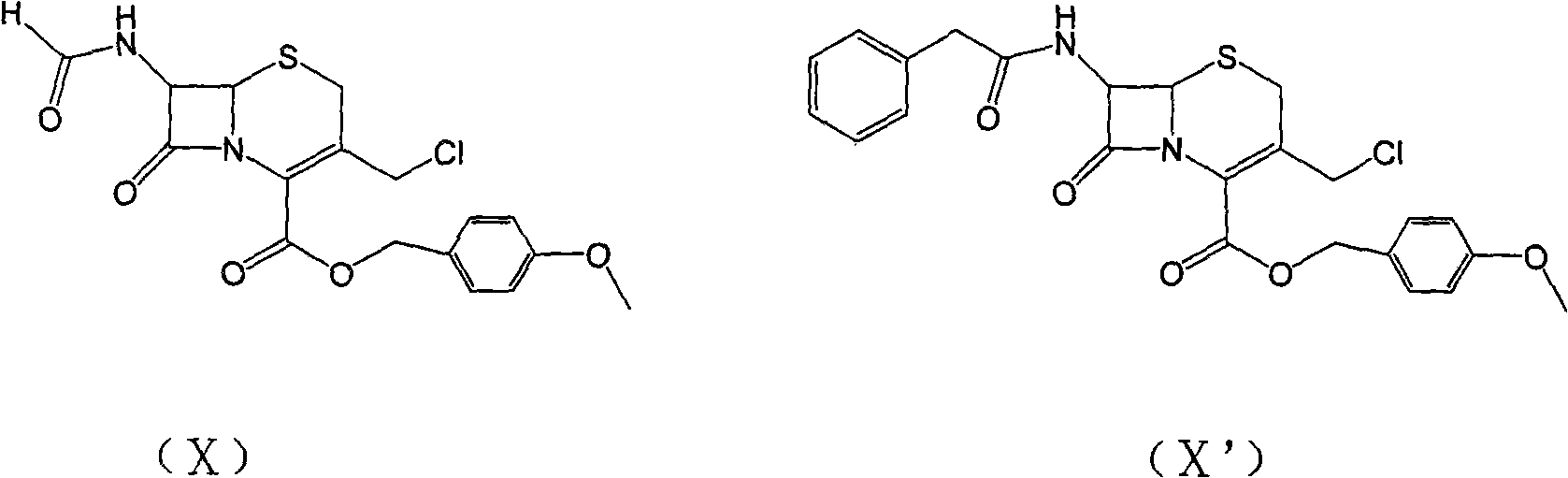
Carbon 2-bit substituted cephalosporin derivative as well as synthetic method and application thereof
A kind of technology of cephalosporins and derivatives, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0120] Synthesis of 7-formylamino-3-(1-methyltetrazolyl-5-mercaptomethyl)-ceph-3-ene-4-carboxylic acid p-methoxybenzyl ester II-1:
[0121]
[0122] Add methylmercaptotetrazolium (3.250g, 28mmol) and 150mL of dichloromethane into the reaction flask, add triethylamine (3.65mL, 28.7mmol) dropwise, and then add formyl (10.012g, 25.2mmol) , After reacting for 4 hours, 11.661 g of white solid was obtained by filtration, and the yield was 97.1%.
Embodiment 2
[0124] Synthesis of 7-formylamino-3-(1-methyltetrazolyl-5-mercaptomethyl)-ceph-3-ene-4-carboxylic acid p-methoxybenzyl ester-1-sulfoxide (III -1):
[0125]
[0126] Add compound (II-1) (9.526g, 20mmol) and 120mL toluene into the reaction flask, add acetic anhydride (21.32mL, 226mmol) and hydrogen peroxide (30%) (10.66mL, 104mmol) under ice-bath conditions, 2h After the reaction was basically completed, saturated sodium bicarbonate solution and sodium bisulfite solution were added to neutralize excess acetic anhydride and hydrogen peroxide, and 8.985 g of white solid was obtained by filtration, with a yield of 91.26%.
Embodiment 3
[0128] 7-Formylamino-3-(1-methyltetrazolyl-5-mercaptomethyl)-2-methylene-ceph-3-ene-4-carboxylic acid p-methoxybenzyl ester-1- Synthesis of sulfoxide (A):
[0129] Compound (III-1) (4.920g, 10mmol), diethylamine (2.05mL, 20mmol), trifluoroacetic acid (1.48mL, 20mmol), formaldehyde (37%) (1.90mL, 20mmol), 11.86mL tert-butyl Alcohol and 45mL of 1,4-dioxane were reacted at 50-53°C for 2h, then the reaction liquid was dropped into 400mL of water, and filtered to obtain 4.526g of khaki solid with a yield of 89.80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com