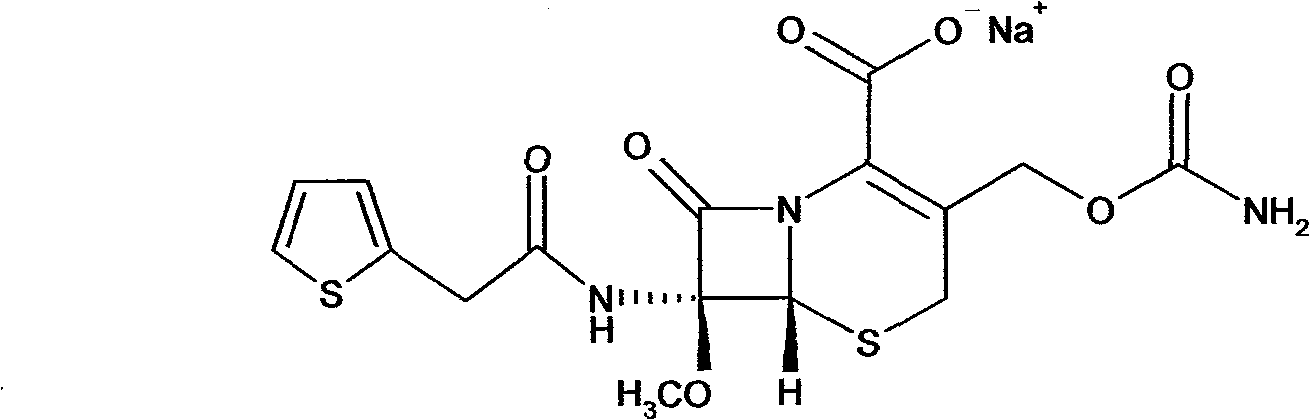
Composition of cefoxitin acid
A technology of cefoxitin and composition, which is applied in the field of composition of antibiotic cefoxitin, and can solve problems such as poor stability, high allergy rate, and high impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] In the aseptic batching workshop, weigh 35.5 kg of sterile cefoxitin acid API and 14.5 kg of arginine API, pour them into a high-efficiency three-dimensional motion mixer, and mix for about 1.5 hours at a speed of 5 rpm. After uniformity, the material is discharged and packaged separately, and the product is obtained.
Embodiment 2
[0016] In the aseptic batching workshop, weigh 35kg of sterile cefoxitin acid API and 15kg of arginine API, pour them into a high-efficiency three-dimensional motion mixer, and mix for about 1.5 hours at a speed of 5 rpm. , discharging, sub-packaging, that is to say.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com