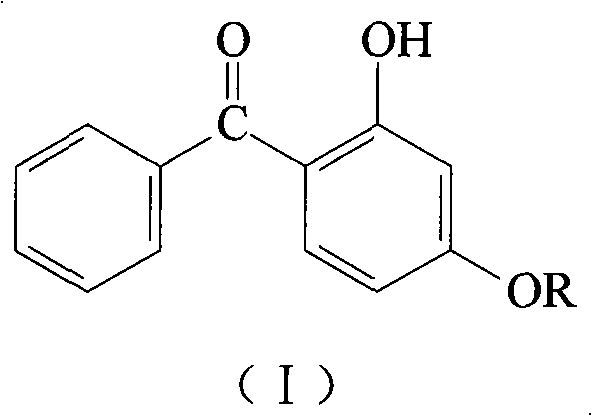
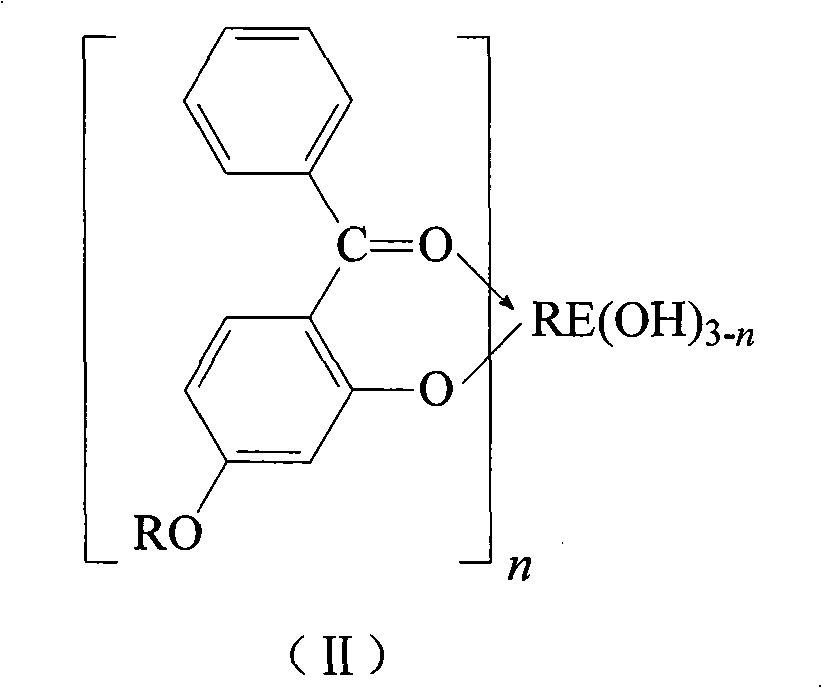
Method for synthesizing o-hydroxy benzophenone-rare earth coordination compound
A technology of o-hydroxybenzophenone and rare earth complexes, applied in the preparation of aldehyde/ketone chelates, organic chemistry, etc., can solve the problems of high production investment and production energy consumption, high production costs, and high solubility, and achieve The effects of production equipment investment and reduction, environmental protection and safety improvement, and production energy consumption reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] At room temperature, 224.2 g of 2-hydroxy-4-methoxybenzophenone (HMBP, 1mol), containing LaCl 3 81.8g (0.333mol) of lanthanum chloride aqueous solution and 40.0g (1mol) of NaOH containing sodium hydroxide aqueous solution, add water until the total mass of the solution is about 1000g, then stir, heat up to 40°C, control the constant temperature reaction for 10min, the reaction forms After filtration, washing and dehydration, the product was dried at 105°C under normal pressure to constant weight to obtain a yellow powder product with a yield of 99.3%. It is determined by analysis that the melting point of the product is >300°C; the contents of La, C and H are 16.77%, 61.49% and 4.68% respectively, which is the same as the chemical formula La(MBP) 3 Consistent (the calculated values of La, C, and H contents are 16.92%, 61.40%, and 4.75%, respectively).
Embodiment 2
[0036] At room temperature, 224.2 g of 2-hydroxy-4-methoxybenzophenone (HMBP, 1mol), containing LaCl 3 122.7g (0.5mol) of lanthanum chloride aqueous solution and 60.0g (1.5mol) of sodium hydroxide aqueous solution containing NaOH, add water until the total mass of the solution is about 1000g, then stir, heat up to 40°C, control the constant temperature reaction for 10min, and react The resultant was filtered, washed with water and dehydrated, then dried at 105°C under normal pressure to constant weight to obtain a yellow powder product with a yield of 99.5%. It is determined by analysis that the melting point of the product is >300°C; the contents of La, C and H are 23.27%, 22.59% and 4.07% respectively, which is the same as the chemical formula La(MBP) 2 (OH) is consistent (the calculated values of La, C, and H contents are 23.06%, 55.79%, and 3.98%, respectively).
Embodiment 3
[0038] At room temperature, 224.2 g of 2-hydroxy-4-methoxybenzophenone (HMBP, 1mol), containing LaCl 3 245.4g (1mol) of lanthanum chloride aqueous solution and 120.0g (3mol) of sodium hydroxide aqueous solution containing NaOH, add water until the total mass of the solution is about 1000g, then stir, heat up to 40°C, control the constant temperature reaction for 10min, the reaction product After filtering, washing with water and dehydration, it was dried at 105°C under normal pressure to constant weight to obtain a yellow powder product with a yield of 99.1%. It is determined by analysis that the melting point of the product is >300°C; the contents of La, C and H are 34.95%, 42.49% and 3.15% respectively, which is the same as the chemical formula La(MBP)(OH) 2 Consistent (the calculated values of La, C, and H contents are 35.07%, 42.41%, and 3.03%, respectively).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com