Paracetamol sustained-release double-layer tablets and preparation method thereof
A technology for acetaminophen and sustained-release tablets, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, and can solve problems such as unclear mechanisms of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
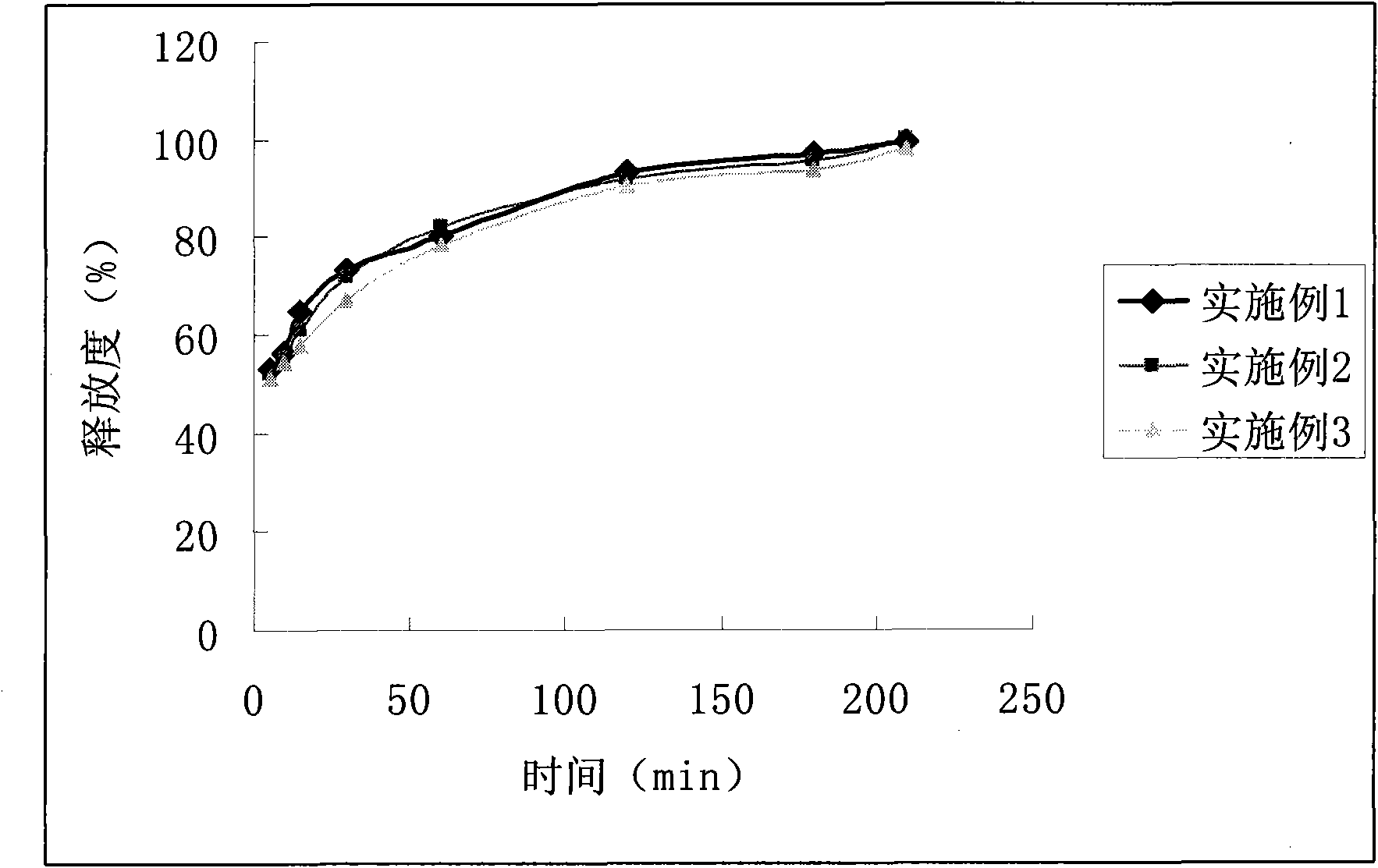
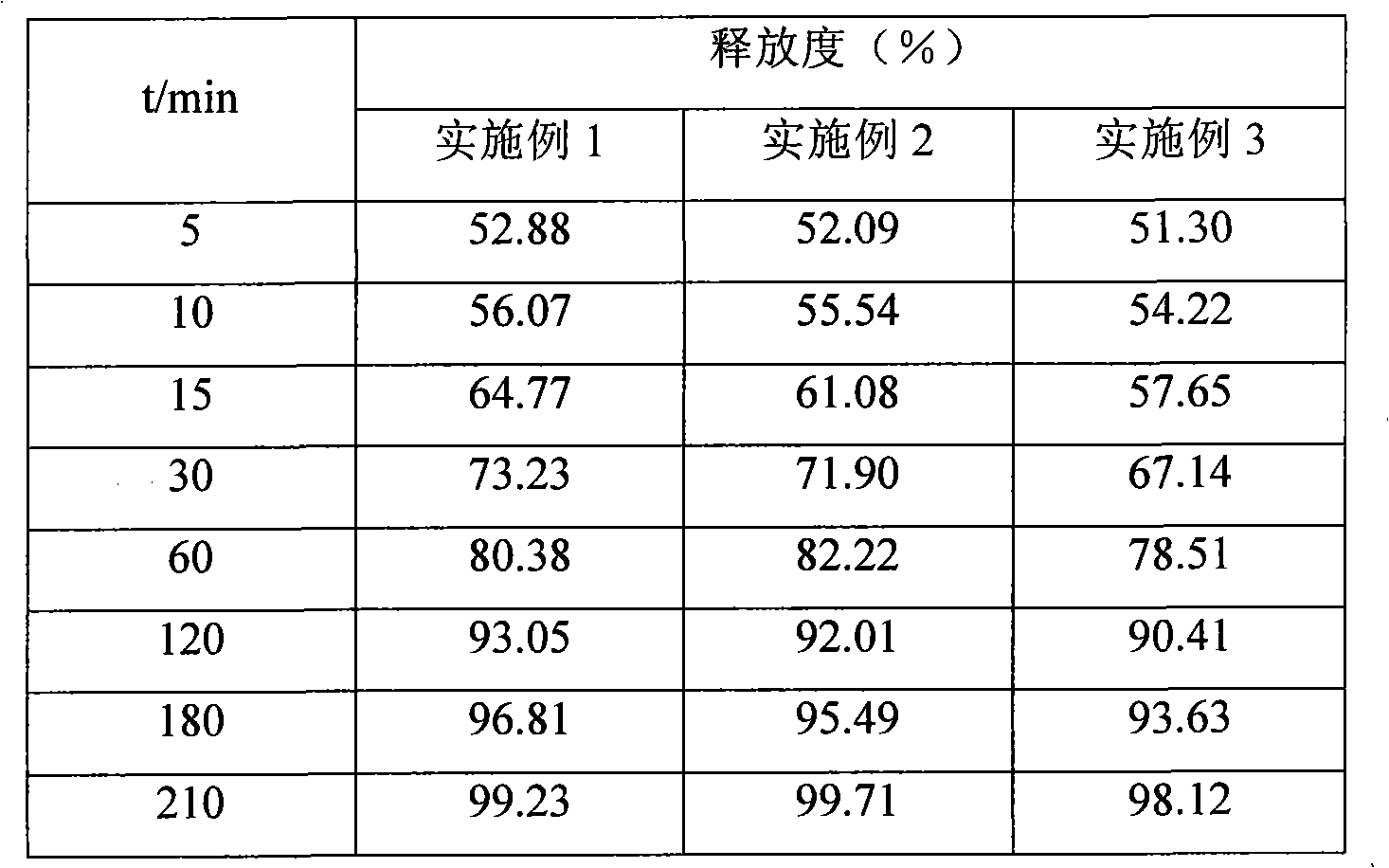
Embodiment 1
[0046] prescription
[0047] Immediate release layer:
[0048] Acetaminophen 325g
[0049] Pregelatinized starch 85g
[0050] Sodium carboxymethyl starch 20g
[0051] 10% Povidone K 30 Aqueous solution
[0052] Magnesium Stearate 6.5g
[0053] Slow release layer:
[0054] Acetaminophen 325g
[0055] Hypromellose K 4 7g
[0056] Microcrystalline Cellulose 42g
[0057] 21g pregelatinized starch
[0058] 10% Povidone K 30 Aqueous solution
[0060] A total of 1000 pieces were made
[0061] Film-forming material:
[0062] Opadry (G-85-white) 20g
[0063] Add pure water to 100ml
[0064] Preparation Process
[0065] (1) Preparation process of plain tablets
[0066] (1.1) Preparation of immediate-release granules
[0067] Pass each component in the prescription through a 100-mesh sieve, and set aside. Fully mix the prescription amount of pregelatinized starch and 1 / 2 amount of carboxymethyl starch sodium, and then mix it with ac...
Embodiment 2
[0075] prescription
[0076] Immediate release layer:
[0077] Acetaminophen 325g
[0078] 40g pregelatinized starch
[0079] Microcrystalline Cellulose 40
[0080] Sodium carboxymethyl starch 20g
[0081] 10% Povidone K 30 Aqueous solution
[0082] Magnesium Stearate 6.5g
[0083] Slow release layer:
[0084] Acetaminophen 325g
[0085] Hypromellose K 4 7g
[0086] Microcrystalline Cellulose 32g
[0087] 32g pregelatinized starch
[0088] 10% Povidone K 30 Aqueous solution
[0089] Magnesium Stearate 6g
[0090] A total of 1000 pieces were made
[0091] Film-forming material:
[0092] Opadry (G-85-white) 20g
[0093] Add pure water to 100ml
[0094] Preparation Process
[0095] (1) Preparation process of plain tablets
[0096] (1.1) Preparation of immediate-release granules
[0097] Pass each component in the prescription through a 100-mesh sieve, and set aside. Fully mix the prescription amount of pregelatinized starch, microcrystalline cellulo...
Embodiment 3
[0105] prescription
[0106] Immediate release layer:
[0107] Acetaminophen 325g
[0108] 30g pregelatinized starch
[0109] Microcrystalline Cellulose 30
[0110] Sodium carboxymethyl starch 20g
[0111] 10% Povidone K 30 Aqueous solution
[0112] Magnesium Stearate 6.5g
[0113] Slow release layer:
[0114] Acetaminophen 325g
[0115] Hypromellose K 4 9g
[0116] Microcrystalline Cellulose 66g
[0117] 10% Povidone K 30 Aqueous solution
[0118] Magnesium Stearate 6g
[0119] A total of 1000 pieces were made
[0120] Film-forming material:
[0121] Opadry (G-85-white) 20g
[0122] Add pure water to 100ml
[0123] Preparation Process
[0124] (1) Preparation process of plain tablets
[0125] (1.1) Preparation of immediate-release granules
[0126] Pass each component in the prescription through a 100-mesh sieve, and set aside. Fully mix the prescription amount of pregelatinized starch, microcrystalline cellulose, and 1 / 2 amount of sodium carboxymeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com