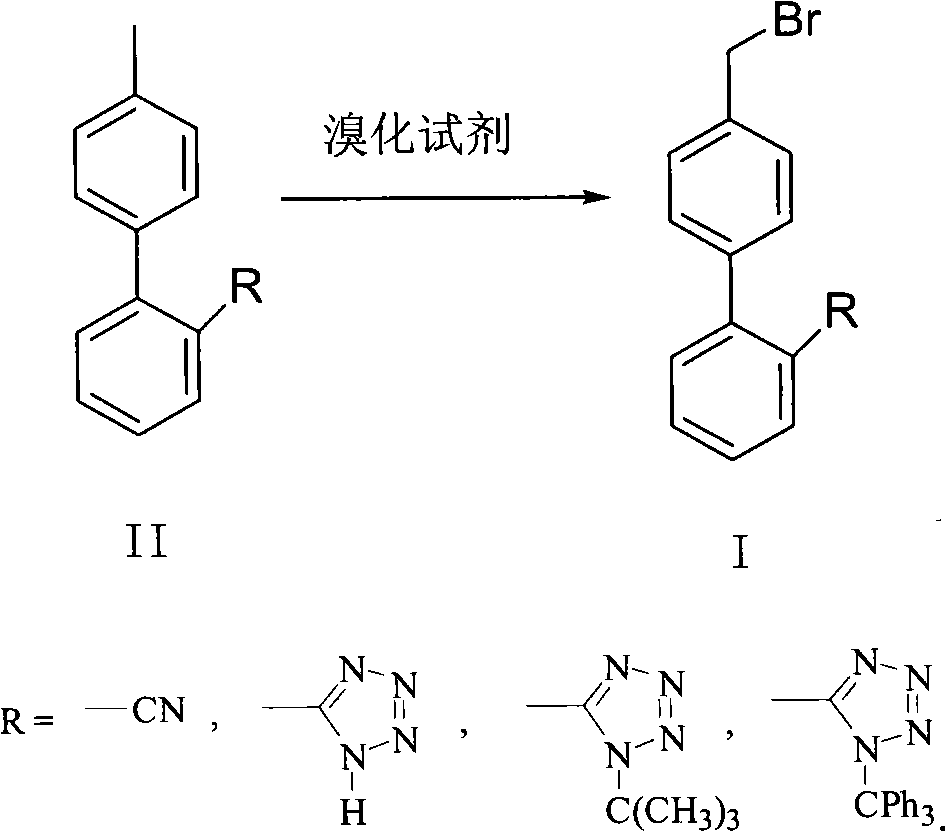
Green synthesis method of bromomethylbiphenyl compound
A technology of bromomethylbiphenyl and compounds, which is applied in the field of preparation of bromomethylbiphenyl compounds, can solve the problem of high energy requirements, and achieve the effects of sufficient production capacity, high yield, and good atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation process 1 of 4'-bromomethyl-2-cyanobiphenyl
[0030] In a 2L three-necked flask, add 96.6g (0.50mol) of 4'-methyl-2-cyanobiphenyl (OTBN), 75g of dibromohydantoin, and 300ml of dichloromethane, and control the temperature in a low-temperature bath to 10-20°C. Then react under sunlight for 2 hours, after the reaction is complete. Detection result (HPLC): Br-OTBN 89.2%, Br 2 -OTBN 6.76%, OTBN 4.04%). The reaction solution was washed with 400ml of 5% sodium bicarbonate and 400ml of water, and the organic layer was concentrated and evaporated to dryness to obtain a crude solid, which was then suspended in 200ml of ethyl acetate and washed with stirring. At ~15°C, heat and crystallize for 4 hours, filter, and dry the solid to obtain 115.6 g, the total yield is 85%, and the purity of the obtained solid is greater than 98%.
Embodiment 2
[0031] Embodiment 2: Preparation process 2 of 4'-bromomethyl-2-cyanobiphenyl
[0032] In a 2L three-necked flask, add 96.6g (0.50mol) of 4'-methyl-2-cyanobiphenyl (OTBN), 94g of dibromohydantoin, and 300ml of dichloromethane, and control the temperature in a low-temperature bath to 0-10°C. Then react under light for 4h, and the reaction is complete. Test result (HPLC): Br-OTBN 91.5%, Br 2 -OTBN 5.05%, OTBN 3.45%). The reaction solution was washed with 400ml of 5% sodium bicarbonate and 400ml of water respectively, concentrated and evaporated to dryness to obtain a crude solid, which was then suspended in 200ml of ethyl acetate and washed with stirring. ℃, keep warm and crystallize for 4 hours, filter, and dry the solid to obtain 117.6 g, the total yield is 86.5%, and the purity of the obtained solid is greater than 98.5%.
Embodiment 3
[0033] Example 3: Preparation process 3 of 4'-bromomethyl-2-cyanobiphenyl
[0034] In a 2L three-necked flask, add 96.6g (0.50mol) 4'-methyl-2-cyanobiphenyl (OTBN), 94g dibromohydantoin, 300ml dichloromethane, add 100ml water, and control the temperature to 0 ~10°C, then reacted for 3 hours under light, and the reaction was completed. Detection result (HPLC): Br-OTBN 91.2%, Br 2 -OTBN 5.95%, OTBN 2.85%). The reaction solution was washed with 400ml of 5% sodium bicarbonate and 400ml of water respectively, concentrated and evaporated to dryness to obtain a crude solid, which was then suspended in 200ml of ethyl acetate and washed with stirring. ℃, heat preservation and crystallization for 4 hours, filter, and dry the solid to obtain 116.2 g, the total yield is 85.4%, and the purity of the obtained solid is greater than 98.5%.
[0035] Embodiment 3: the preparation process of N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl)tetrazolium (BBTT) crude product
[0036] In a 500...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com