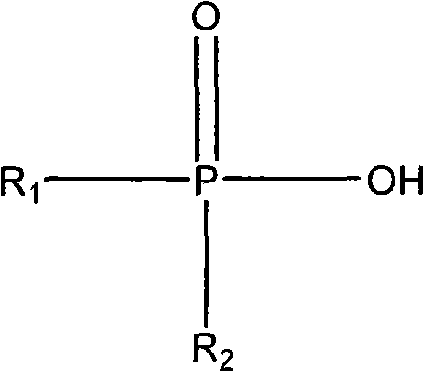
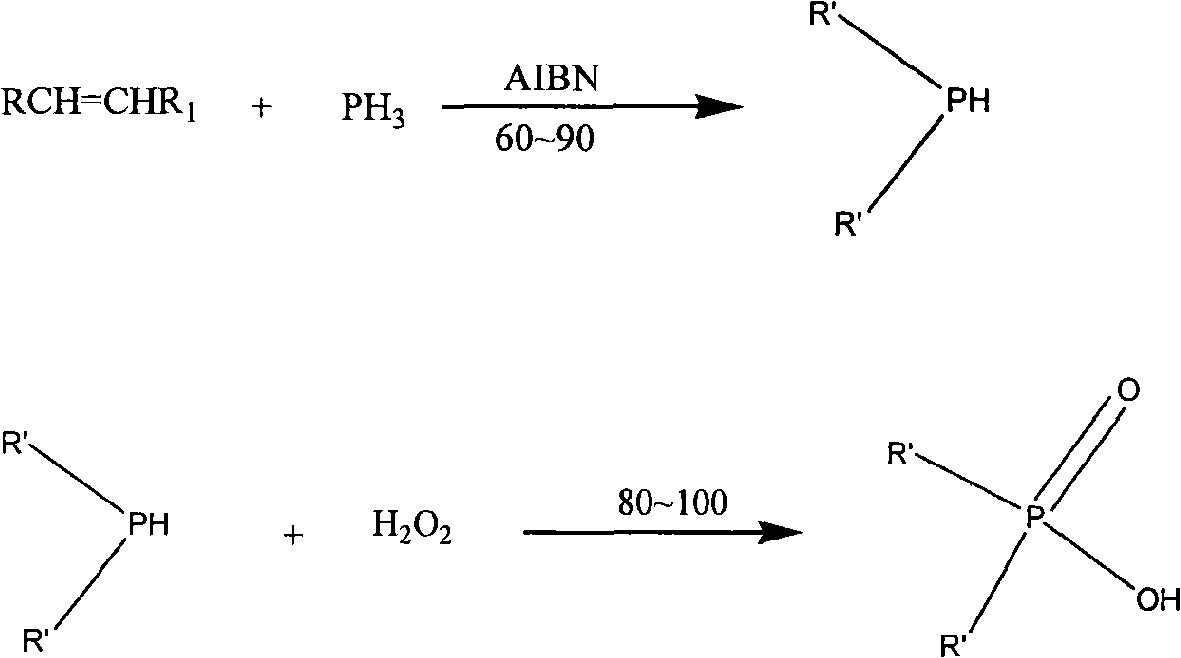
Synthesis method of dialkyl phosphinic acid extractant
A technology of dialkyl phosphinic acid and synthesis method, applied in the field of synthesis of dialkyl phosphinic acid extractant, can solve the problems of dispersion, inability to meet the supply, scarce metal burial amount, etc., and achieve high selectivity and extraction effect Good, easy to synthesize effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of two (2,7-dimethyloctyl) phosphinic acid
[0030] Weigh 1.5g of azobisisobutyronitrile and 50g of acetic acid to prepare a solution, dissolve it and add it to the dropping device. Weigh 42.4g (0.4mol) of sodium hypophosphite monohydrate and 168g (1.2mol) of 2,7-dimethyloctene into a four-neck flask, add acetic acid as a solvent, stir until the salt dissolves, and start heating. Nitrogen is continuously fed into the reaction system, and the flow rate is controlled to prevent unreacted olefins from being taken out of the reaction phase. After the temperature rose to about 96°C, reflux began to appear in the condenser. At this temperature, evenly add the catalyst solution dropwise, control the reflux, and react for 16 hours. The reaction solution was cooled down to be processed.
Embodiment 2
[0031] Example 2 Preparation of two (2,3 dimethylheptyl) phosphinic acid
[0032] Weigh 1.5g of azobisisobutyronitrile and 50g of acetic acid to prepare a solution, dissolve it and add it to the dropping device. Weigh 42.4g (0.4mol) of sodium hypophosphite monohydrate and 151g (1.2mol) of 2,3-dimethylheptene into a four-necked flask, and add acetic acid as a solvent, stir until the salt dissolves and start heating. Nitrogen was continuously fed into the reaction system. After the temperature rose to about 94°C, reflux began to appear in the condenser. At this temperature, evenly add the catalyst solution dropwise, control the reflux, and react for 16 hours.
Embodiment 3
[0033] Example 3 Preparation of two (2-n-butylhexyl) phosphinic acid
[0034] Weigh 1.5g of azobisisobutyronitrile and 50g of acetic acid to prepare a solution, dissolve it and add it to the dropping device. Weigh 42.4g (0.4mol) of sodium hypophosphite monohydrate and 168g (1.2mol) of 2-n-butylhexene into a four-necked flask, and add acetic acid as a solvent, stir until the salt dissolves and start heating. Nitrogen was continuously fed into the reaction system. After the temperature rose to about 95°C, reflux began to appear in the condenser. At this temperature, evenly add the catalyst solution dropwise, control the reflux, and react for 16 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com