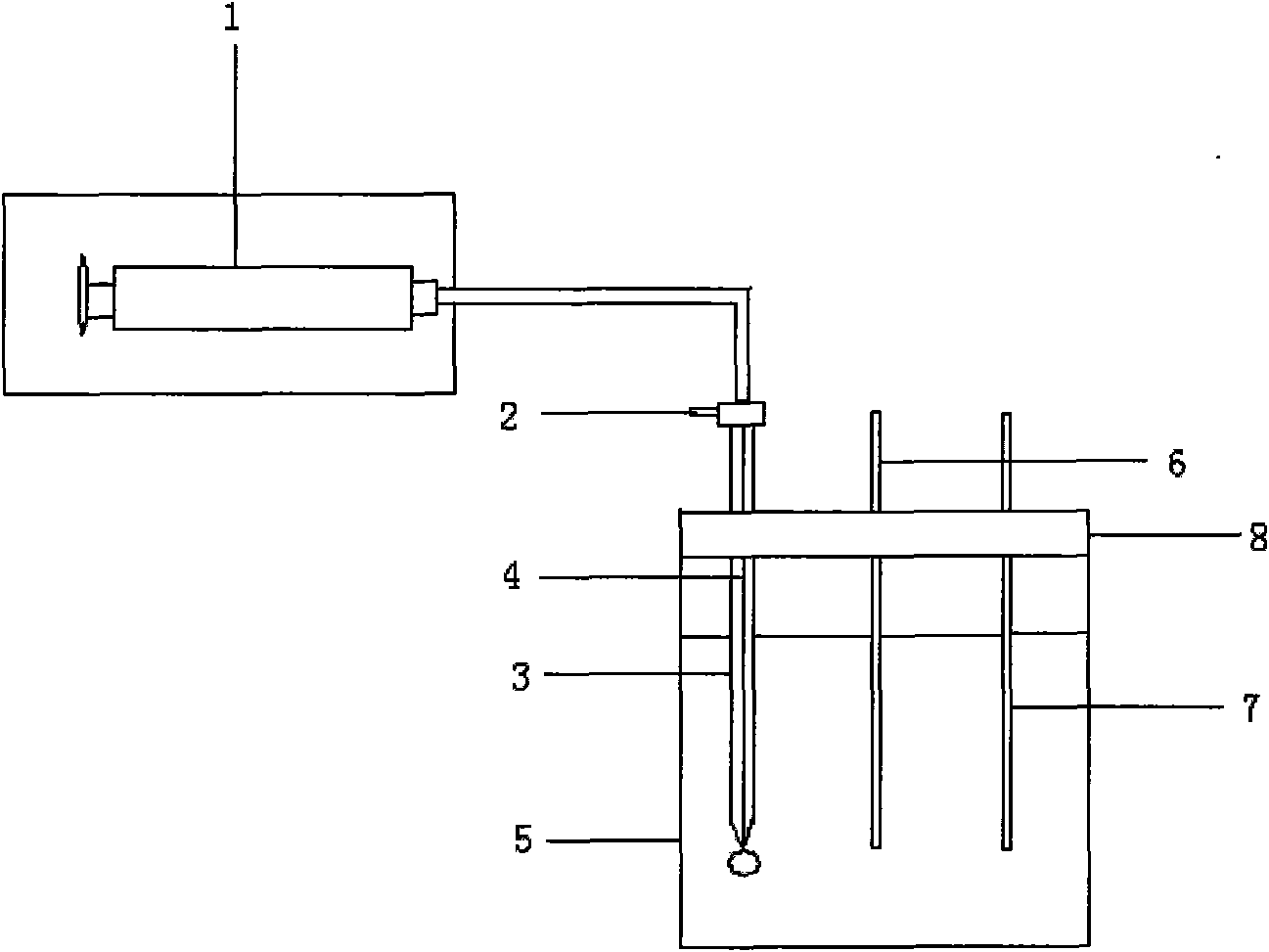
Chemical dropping electrode and method for monitoring pH value in solution
A dripping electrode and chemical technology, applied in the field of electrochemistry, can solve the problems of long-term environmental impact on the health of operators, inability to reuse, highly toxic metal mercury, etc., to avoid electrode contamination, easy calculation, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Influence of Ionic Liquid Concentration in Organic Phase on pH Detection
[0021] Prepare 0.01mol.L -1 Lithium chloride solution 20mL, use hydrochloric acid and sodium hydroxide to adjust the pH of the lithium chloride solution to 2; place the three-phase electrode on the electrode holder, use a syringe to draw the solution containing different concentrations of 1-n-butyl 3-methylimidazole hexafluoro Phosphate 1,2-dichloroethane, control the droplet size at the top of the glass dropper to 11.11 μL, use the square wave method to scan within -1 ~ 1V, the results are shown in Table 1. With the increase of the concentration of the ionic liquid, the peak current of the hydrogen ion also increases under the condition of the same pH value, so the concentration of the ionic liquid is fixed in the process of measuring the pH.
[0022] Table 1
[0023] Ionic liquid concentration / mol.L -1
Embodiment 2
[0025] Peak current obtained at different pH values
[0026] Prepare 0.01mol.L -1 Lithium chloride solution 20mL, use hydrochloric acid and sodium hydroxide to adjust to different pH as the solution to be tested; place the three-phase electrode on the electrode holder, use a syringe to draw 1.25mol.L -1 1,2-dichloroethane of 1-n-butyl 3-methylimidazolium hexafluorophosphate, control the droplet size at the top of the glass dropper to 11.11 μL, and use the square wave method to carry out within -1 ~ 1V The scan results are shown in Table 2. As the pH value increases, the peak current has a linear relationship with the pH, so under a certain background, the pH of the solution can be obtained through the magnitude of the peak current in the solution.
[0027] Table 2
[0028] pH
Embodiment 3
[0030] Influence of droplet size on the detection effect of pH value
[0031] Prepare 0.01mol.L -1 Lithium chloride solution 20mL, use hydrochloric acid and sodium hydroxide to adjust the pH to 2.5 as the solution to be tested; place the three-phase electrode on the electrode holder, and use a syringe to draw 1.25mol.L -1 1,2-dichloroethane of 1-n-butyl 3-methylimidazolium hexafluorophosphate, control the droplet at the top of the glass dropper to be of different sizes, use the square wave method to scan within -1 ~ 1V , the results are shown in Table 3. During the scanning process, the droplet size of the working electrode will also affect the magnitude of the peak current, so the size of the working electrode must be fixed during the measurement of pH.
[0032] table 3
[0033] Droplet size / μL
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com