Canine distemper live vaccine and preparation method thereof
A technology for live vaccines and canine distemper, which is applied in pharmaceutical formulas, medical preparations containing active ingredients, antibody medical ingredients, etc., and can solve problems such as immune failure, uneven immunogenicity of virus strains, and low antigen titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
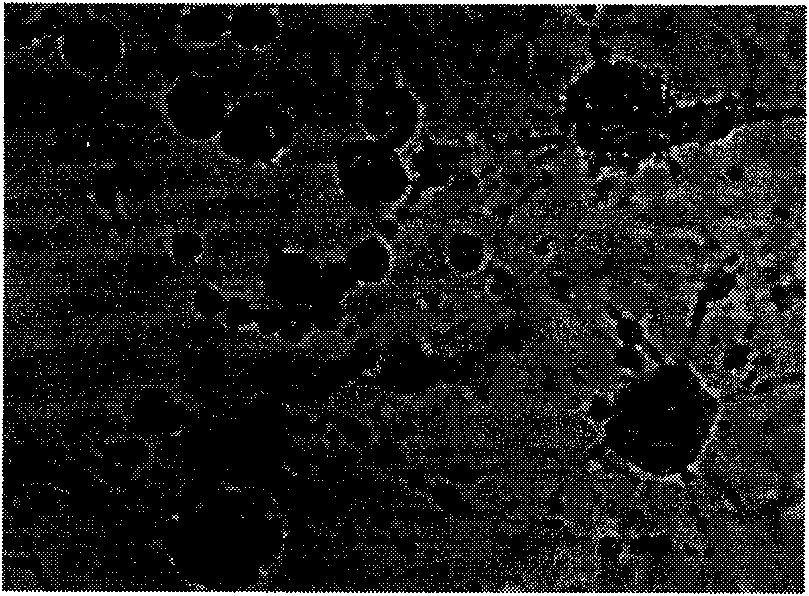
[0051] Purity Test of Virus Seeds
[0052] 1 Sterility test Inoculate the virus culture into two tubes of T.G, G.P small tubes and G.A slant medium respectively, each tube is 0.2ml, one is cultured at 37°C; one is cultured at 25°C, and observed for 3 days, the virus after transplantation The T.G, G.P small tubes and G.A slant medium inoculated with the cultures were observed for 5 days without bacteria and mold growth. Whether there is bacteria and mold growth.
[0053] 2. Mycoplasma test Take 5ml of virus culture, inoculate it into a vial of liquid mycoplasma culture medium, then get 0.8ml from the suspension in the vial and transplant it into 2 small test tubes of liquid mycoplasma culture medium and 2 pairs of agar solid plates. Liquid cultures were cultured at 37°C, solid cultures were placed at 37°C with 5% CO 2 Environmental cultivation. After culturing for 5, 10, and 15 days, take 0.6-0.8ml of the culture from the vial and transplant it into 2 small test tubes of liq...
Embodiment 2
[0059] manufacture of vaccines
[0060] 1 Preparation of cell nutrient solution
[0061] Conventional cell growth solution (containing 10% primary newborn bovine serum) and maintenance solution (containing 2% primary newborn bovine serum) were used for cell culture.
[0062] 2 Preparation of seed poison for production
[0063] Use the isolated natural attenuated strain of canine distemper to inoculate the Vero cell monolayer on the spinner bottle, the amount of inoculation is 2% of the maintenance solution, and it can be harvested when the cell lesion reaches 80%, and then passed on for one generation to prepare the production Use poison.
[0064] 3 Propagation of virus fluid
[0065] The seed virus for production was used to inoculate the Vero cell monolayer on the spinner bottle, and the inoculation amount was 2% of the cell maintenance solution. Then the spinner bottle cells were cultured at 37°C with a rotation speed of 8-10r / h.
[0066] After inoculation, observe the...
Embodiment 3
[0071] Finished product testing of vaccines.
[0072] (1) Sterility test should be sterile growth. According to the "Chinese Veterinary Pharmacopoeia" in the inspection.
[0073] (2) Mycoplasma inspection shall be carried out according to the regulations in "Chinese Veterinary Pharmacopoeia", and there should be no mycoplasma growth.
[0074] (3) Identification test The vaccine is 10 -2 diluted, with an equivalent of 10 -1 Diluted canine distemper positive serum was mixed, neutralized at 37°C for 30 minutes, inoculated into 4 bottles of Vero cells, and set up 2 bottles of virus control group at the same time, cultured at 37°C, and observed CPE continuously for 96-120 hours.
[0075] (4) Exogenous virus inspection shall be carried out according to the regulations in "Chinese Veterinary Pharmacopoeia", and there should be no exogenous virus.
[0076] (5) Safety inspection After dissolving each dose of vaccine with 1ml of normal saline, intramuscularly inoculate 5 susceptible...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com