Buprenorphine patch
A technology of buprenorphine hydrochloride and patch, which is applied in the direction of medical preparations of non-active ingredients, sheet delivery, nervous system diseases, etc. It can solve the problems of long-term administration, unsatisfactory patients, and unsatisfactory effects, etc. problem, to achieve the effect of good drug stability, great application value, and good percutaneous absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3、 comparative example 1~5
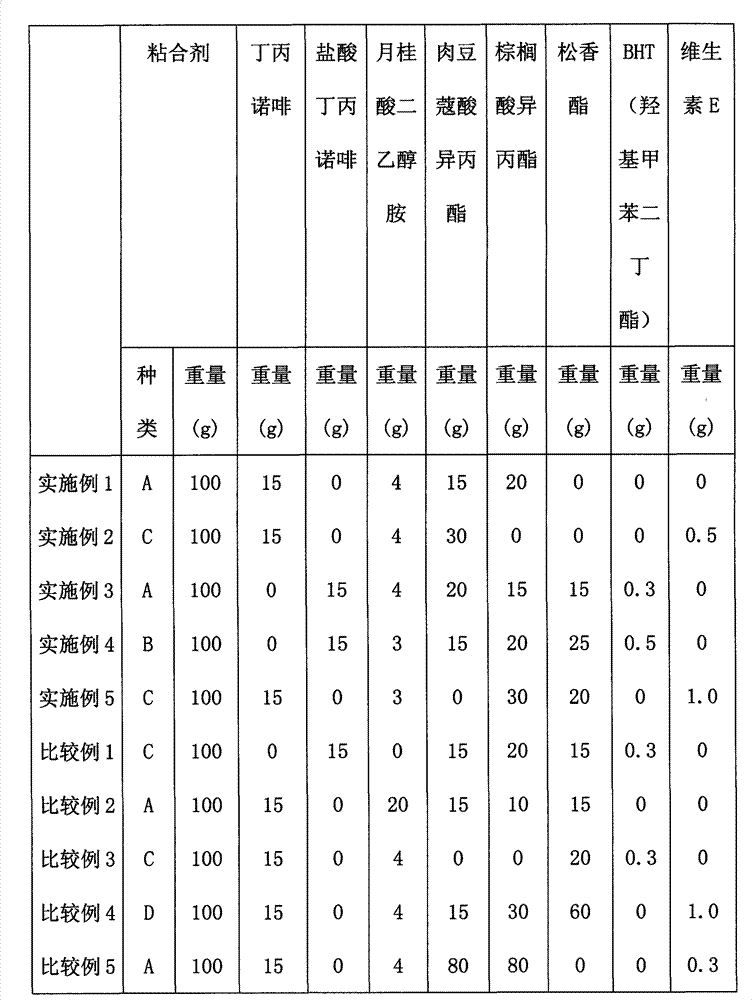
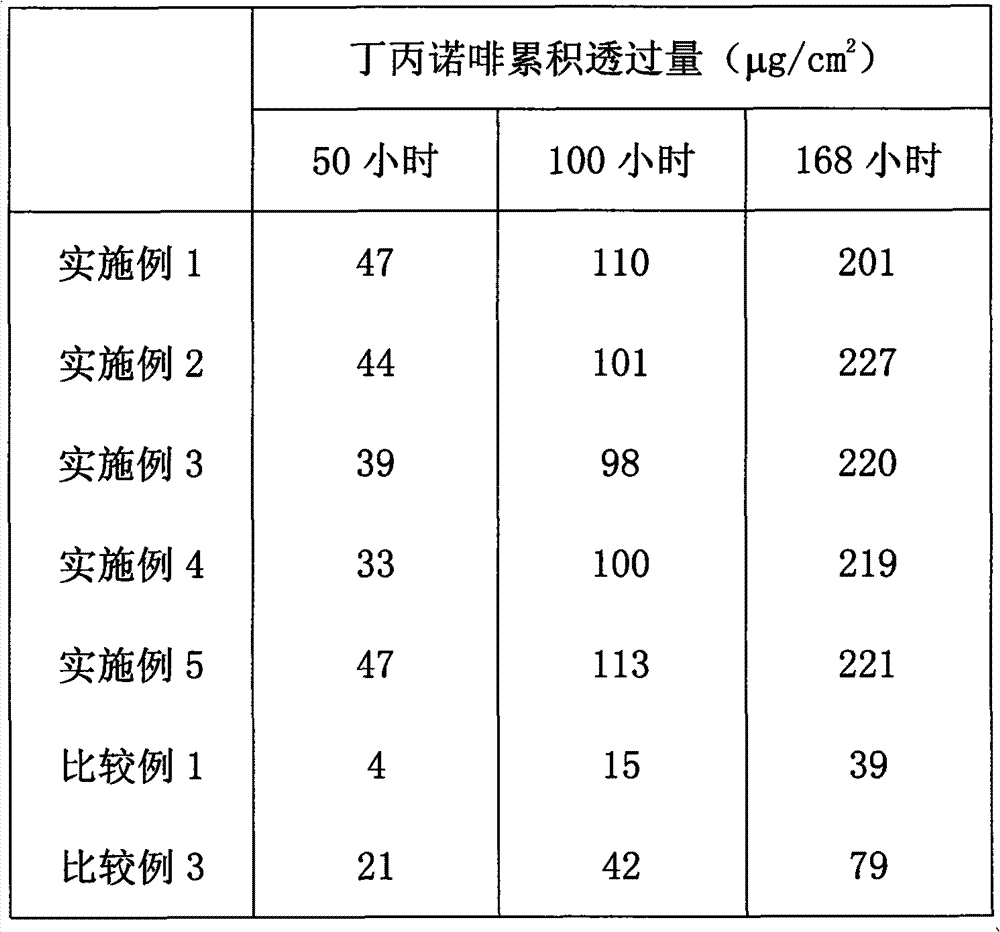
[0026] According to the proportion shown in Table 1, add buprenorphine, buprenorphine hydrochloride, diethanolamide laurate, isopropyl myristate, isopropyl palmitate and rosin ester (Arakawa chemical Co., Ltd., rosin ester GA-90AF), uniformly mixed to form a solution for transdermal absorption, and coated on a polyethylene terephthalate (PET) film to control the thickness of the coating film so that the thickness of the film after drying is 100 μm , dried at a temperature of 60° C. for 8 hours, and the transdermal absorption patch was prepared after the solvent evaporated.
[0027] Table 1
[0028]
[0029] In the above table, A is adhesive A; B is adhesive B; C is adhesive DUR-TAC 87-2677; D is adhesive: MASCOS10
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com