Danshensu derivative, preparation method thereof, and application thereof in pharmacy
A technology of danshensu derivatives and reaction, which is applied in the field of synthesis of danshensu derivatives, can solve problems such as inappropriate chiral resolution, and achieve the effects of reducing leakage rate, increasing SOD activity, and reducing MDA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] According to the following reaction scheme, prepare 2-acetoxy-3-(6-nitro-3,4-methylenedioxophenyl)propionic acid (1):
[0025]
[0026] Take raw material 3,4-methylene oxyphenyl lactic acid 0.5g, 2.4mmol dissolved in 6mL acetic acid and 1.5mL acetic anhydride mixture, put in ice bath, 1 drop of concentrated nitric acid, 21 drops of acetic acid, 21 drops of acetic anhydride Prepare a mixed solution, slowly drop it into the reaction solution, react in an ice-water bath for 4 hours, use petroleum ether / ethyl acetate / acetic acid (V / V / V) = 15:15:3 as the developer, and display under TLC ultraviolet light Raw materials (R f =0.5), the main product point in the reaction solution (R f =0.5), the raw material can be roasted brown after soaking in phosphomolybdic acid, and the two products are baked light yellow, stop the reaction, add 15mL water, extract with ethyl acetate, wash the extract with water 3 times, dry over anhydrous magnesium sulfate, Mix crude silica gel, sepa...
Embodiment 2
[0027] Example 2 Preparation of 2-hydroxyl-3-(6-nitro-3,4-methylenedioxophenyl) propionic acid (2)
[0028] Mix 1mL of concentrated nitric acid and 1.4mL of concentrated sulfuric acid to make a mixed acid solution, put it in an ice-water bath, dissolve 0.2g, 0.95mmol of raw material 3,4-methylene oxide phenyl lactic acid in 1mL of acetone, slowly drop into the mixed acid under stirring , yellow smoke was emitted during the dropwise addition process, stirred and reacted in an ice-water bath for 10 minutes, using petroleum ether / ethyl acetate / acetic acid (V / V / V)=10:10:2 as the developer, TLC showed that the raw material (R f =0.7), there are two product points in the reaction solution (R f =0.5, 0.3), stop the reaction, pour the reaction solution into 20mL water, extract with ethyl acetate, dry the extract, filter the desiccant, mix with crude silica gel, separate and purify by column chromatography, the eluent is petroleum ether / ethyl acetate Ester / acetic acid (V / V / V)=60:20:1,...
Embodiment 3
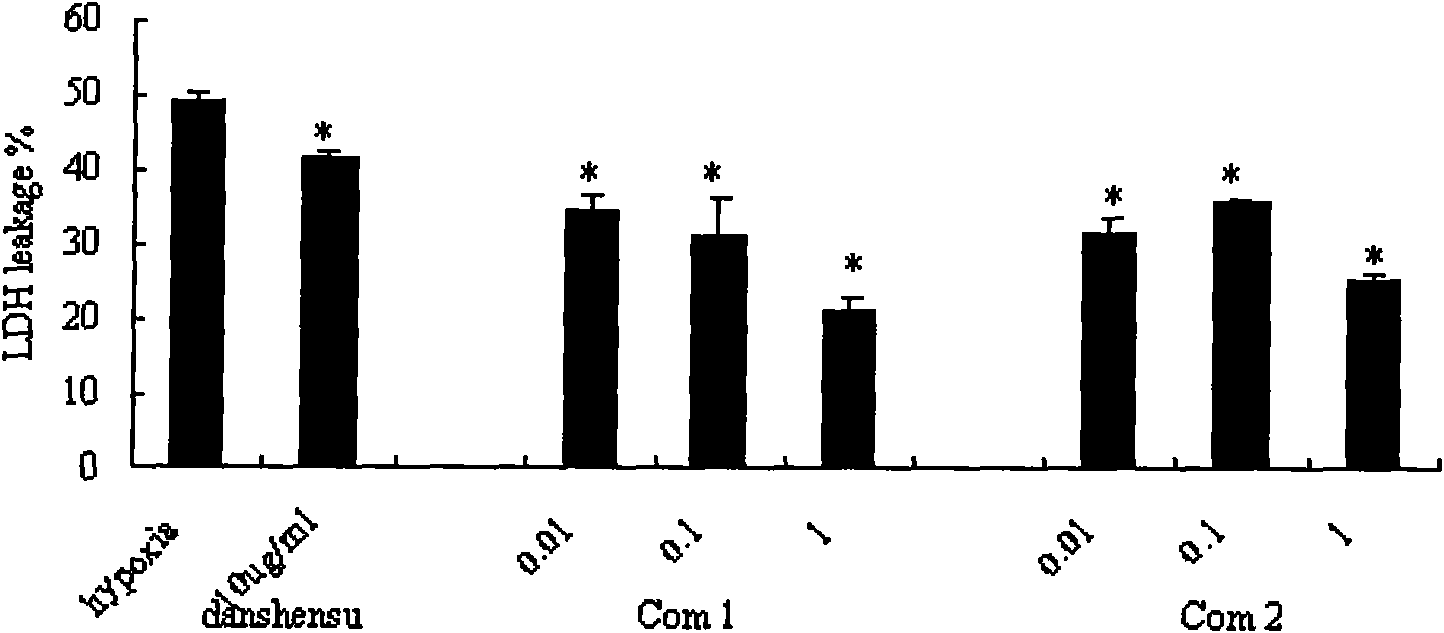
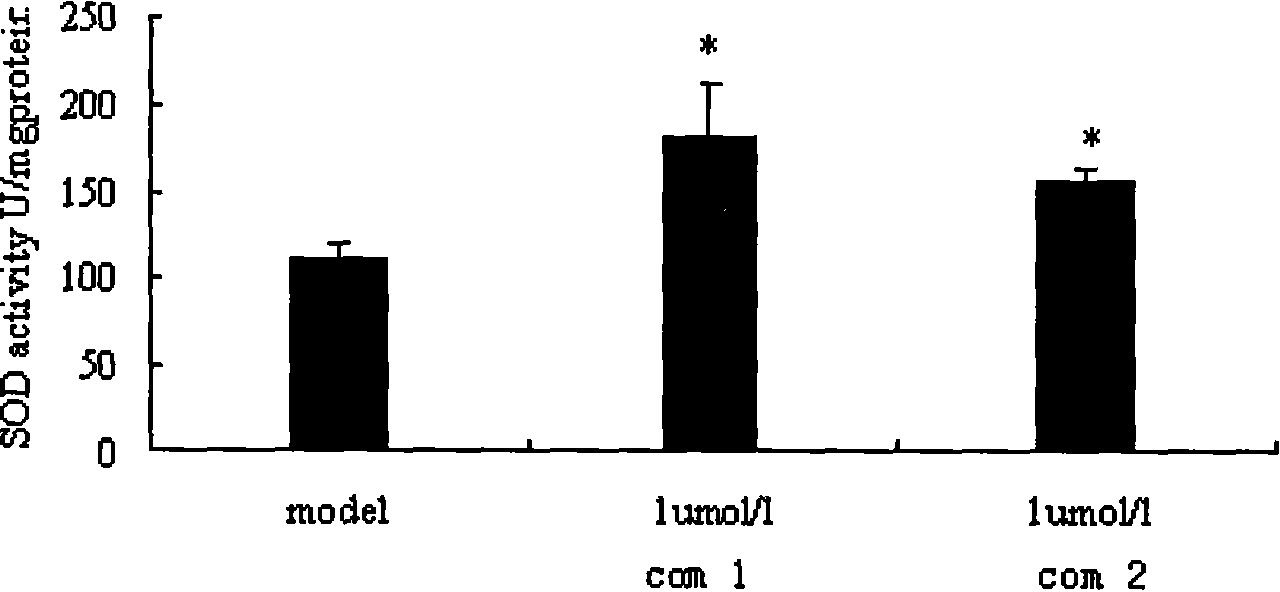
[0030] Example 3 Experiments on Danshensu Derivatives' Protective Effect on Ischemic Myocardium
[0031] Cardiomyocyte hypoxia model was established by primary culture of neonatal rat cardiomyocytes according to conventional methods. The experiment was divided into normal control group: no drug intervention, no hypoxia and glucose deficiency, model group: no drug intervention, hypoxia and glucose deficiency for 5 hours, medication group: drug administration for 10 hours -8 mol / L, 10 -7 mol / L, 10 -6 mol / L, hypoxia and glucose deficiency.
[0032] The experimental results show that the intracellular LDH of the normal control group is 100%, and the difference between the intracellular LDH of each medication group and the hypoxia group and the normal control group is the LDH leakage rate. Compared with the hypoxia group, the compound of the present invention can reduce the hypoxia Cardiomyocyte LDH leakage rate ( figure 1); Compared with the hypoxic group, the compound of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com