Method for preparing magnesia by taking magnesium chloride containing brine as raw material
A technology of magnesium chloride and magnesium oxide, which is applied in the field of salt chemical industry, can solve the problems of difficult control of the reaction process and unstable product quality, and achieve the effects of good crystal shape, reduced energy consumption, and improved environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
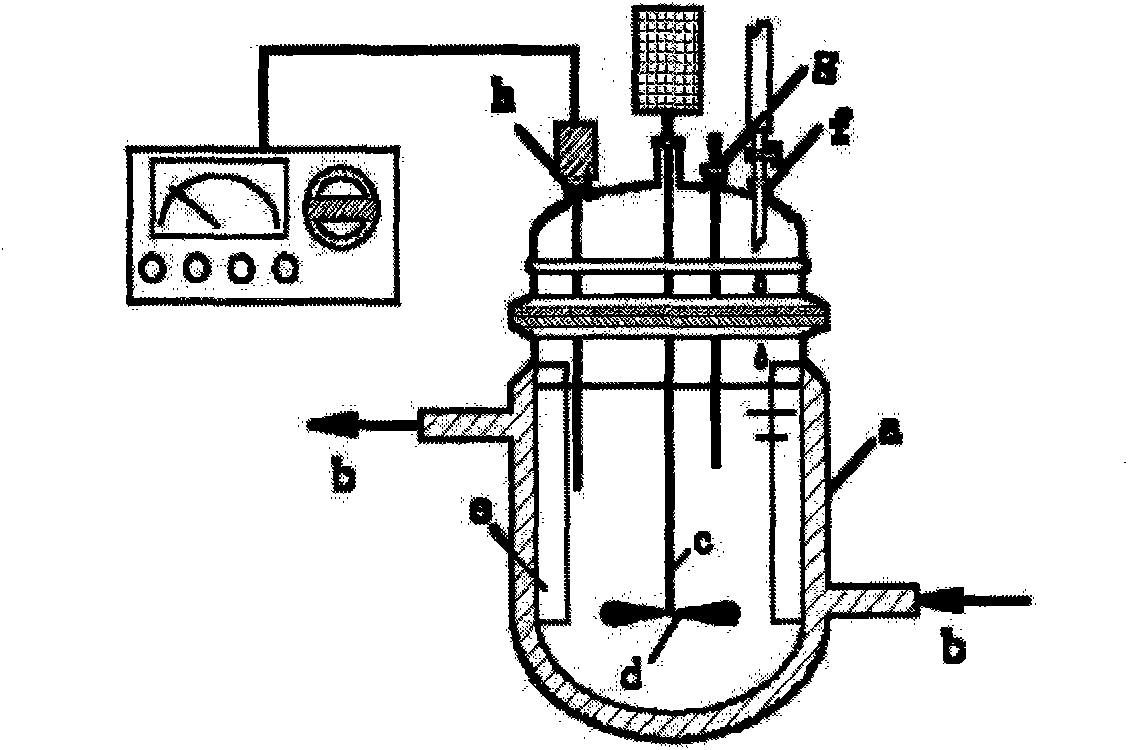
[0070] See figure 2 . The jacketed reaction tank used is provided with a circulating water inlet and outlet b on the upper and lower parts of the outer wall of the tank a, one end of the stirring shaft c is connected to the external motor of the tank, and the other end extends into the tank. Stirring blades d are installed on the stirring shaft c in the body, four baffles e are fixedly installed on the inner wall of the tank, and the dripping nozzle f, thermometer socket g, and pH meter socket h are opened on the upper part of the tank body.
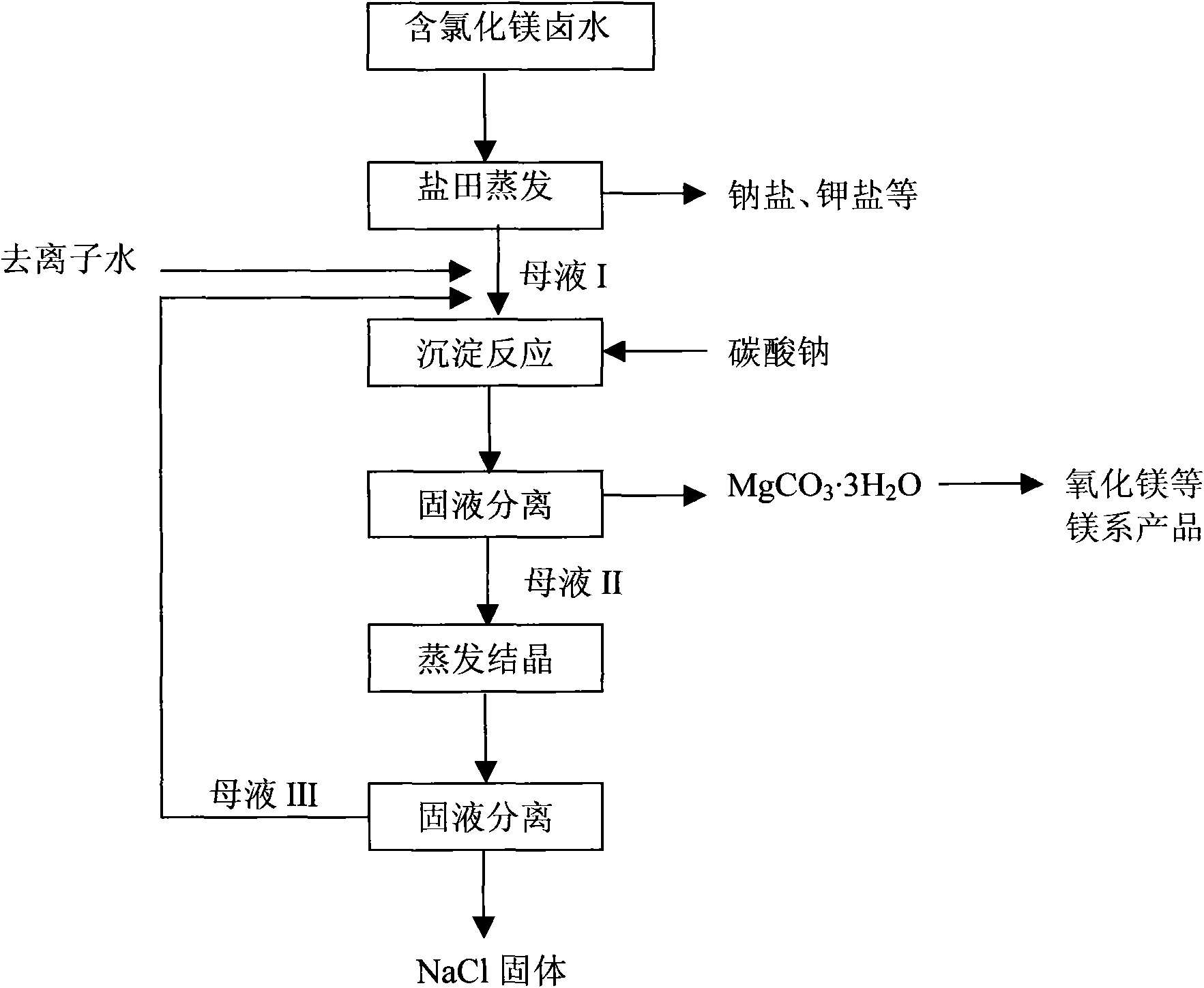
[0071] Process flow of the present invention please refer to figure 1 .
[0072] 1) The original brine is naturally evaporated and concentrated in the salt field, and sodium chloride, potassium chloride, potassium sulfate, magnesium sulfate, magnesium potassium double salt, etc. are crystallized respectively, and crystallization mother liquor I containing magnesium chloride is obtained simultaneously;
[0073] 2) The magnesium chlori...
Embodiment 2
[0078] The jacketed reaction tank used in Example 1 was used.
[0079] 1) The original brine is naturally evaporated and concentrated in the salt field, and sodium chloride, potassium chloride, potassium sulfate, magnesium sulfate, magnesium potassium double salt, etc. are crystallized respectively, and a crystallization mother liquor containing magnesium chloride is obtained at the same time;
[0080] 2) Precipitate the crystallization mother liquor containing magnesium chloride obtained in step 1), filter to remove impurities and crystals, and obtain refined brine with a magnesium chloride content of 4.5mol / L;
[0081] 3) The refined brine containing magnesium chloride obtained in step 2) is adjusted with deionized water, and is prepared with 2000ml of magnesium chloride reaction bottom liquid with an initial concentration of 2mol / L, and the pH value of the magnesium chloride reaction bottom liquid is adjusted to 3 with dilute hydrochloric acid;
[0082] 4) the magnesium chl...
Embodiment 3
[0085] The jacketed reaction tank used in Example 1 was used.
[0086] 1) The original brine is naturally evaporated and concentrated in the salt field, and sodium chloride, potassium chloride, potassium sulfate, magnesium sulfate, magnesium potassium double salt, etc. are crystallized respectively, and a crystallization mother liquor containing magnesium chloride is obtained at the same time;
[0087] 2) Precipitating the crystallization mother liquor containing magnesium chloride obtained in step 1), filtering to remove impurities and crystals, and obtaining refined brine with a magnesium chloride content of 4mol / L;
[0088]3) The refined brine containing magnesium chloride obtained in step 2) is adjusted with deionized water to prepare 2000ml of magnesium chloride reaction bottom solution with an initial concentration of 3mol / L, and the pH value of the magnesium chloride reaction bottom solution is adjusted to 2 with dilute hydrochloric acid;
[0089] 4) the magnesium chlor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com