Method for preparing 2-ethylhexyl chloroformate
A technology of ethylhexyl and chloroformic acid, which is applied in the preparation of 2-ethylhexyl chloroformate and the field of preparation of 2-ethylhexyl chloroformate, can solve the problem of unfavorable 2-ethylhexyl chloroformate Ester synthesis, storage, transportation, dangerous operation, difficult control and operation of liquid phosgene, etc., to achieve the effect of low toxicity, convenient weighing and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Solid phosgene (77g), sodium carbonate (55g), and catalyst N,N-dimethylformamide (10g) were mixed uniformly, and stirred in toluene (750mL) at 0°C for 30 minutes. Then, 750 mL of 2-ethylhexanol / toluene solution with a concentration of 0.69 mol / L was slowly dropped into the mixture for 30 minutes. After the reaction system was stirred at 0° C. for 8 hours, sodium carbonate was separated by suction filtration, and then the solvent was separated by distillation under reduced pressure to obtain 89 g of a colorless oily liquid, namely 2-ethylhexyl chloroformate, with a yield of 93.2%. The purity of 2-ethylhexyl chloroformate obtained by titration analysis was 98%.
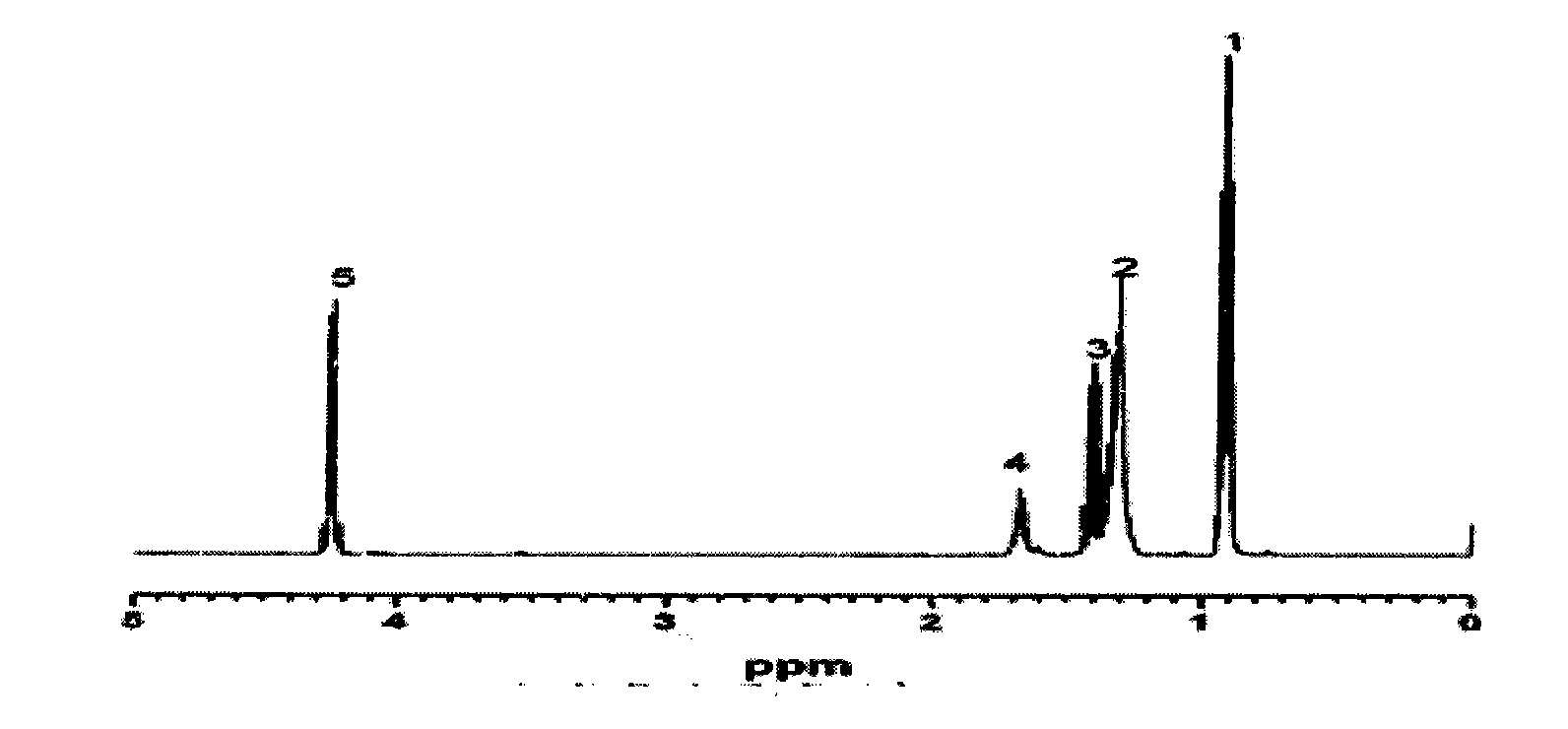
[0020] The proton nuclear magnetic spectrum of product 2-ethylhexyl chloroformate is as figure 1 , the chemical shift of the chemical environment of the hydrogen on 2-ethylhexyl chloroformate is located at δ0.93[6H, CH 3 (1)], 1.33 [6H, CH 2 (2)], 1.41 [2H, CH 2 (3)], 1.65[1H, CH(4)], 4.2[2H, CH 2 (5)]. The...
Embodiment 2
[0022] Solid phosgene (77g), anhydrous sodium bicarbonate (44g), and catalyst triethylamine (13.1g) were mixed uniformly, and stirred in dichloromethane (750mL) at 0°C for 30 minutes. Then, 750 mL of 2-ethylhexanol / dichloromethane solution with a concentration of 0.69 mol / L was slowly dropped into the mixture for 30 minutes. After the reaction system was stirred at 0°C for 8 hours, sodium bicarbonate was separated by suction filtration, and then the solvent was separated by distillation under reduced pressure to obtain 87 g of a colorless oily liquid, namely 2-ethylhexyl chloroformate, with a yield of 91.1%. , the purity obtained by titration analysis of 2-ethylhexyl chloroformate is 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com