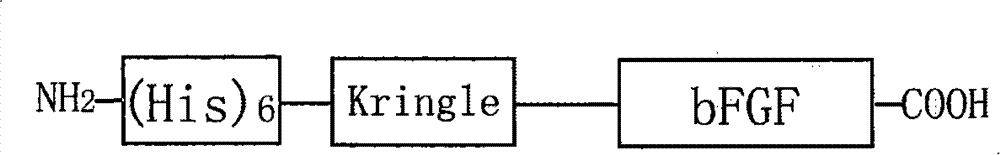
Recombined protein specially combined with fiber protein and application thereof
A recombinant protein and protein technology, which is applied to the recombinant protein specifically binding to fibrin and its application field, can solve the problems of low accumulation of recombinant bFGF and difficult sustained action of drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, preparation recombinant K1bFGF and K4bFGF protein
[0048] 1) Acquisition of Kringle1 and Kringle4
[0049] The Kringle1 fragment was amplified by complementary sequence binding of primers K1-1, K1-2, K1-3, K1-4 and K1-5.
[0050] The order of adding primers is: K1-1 and K1-2 are used for the first amplification, K1-3 and K1-4 are used for the second time, and K1-1 and K1-5 are used for the third time.
[0051] The sequences of primers K1-1, K1-2, K1-3, K1-4 and K1-5 are as follows:
[0052] K1-1: 5’-AGA GGG ACG ATG TCC AAA ACA AAA AAT GGC ATC ACC TGT CAA AAATGG AGT TCC ACT TCT CCC CAC AGA CCT AGA TTC TCA CCT-3’;
[0053] K1-2: 5'-CCT GCG GAT CGT TGT CTG GAT TCC TGC AGT AGT TCT CCT CCA GTCCCT CTG AGG GGT GTG TAG CAG GTG AGA ATC TAG GTC TGT-3';
[0054] K1-3: 5'-TAT CTC TCA GAG TGC AAG ACT GGG AAT GGA AAG AAC TAC AGAGGGACG ATG TCC AAA AC-3';
[0055] K1-4: 5'-CAT ATC TCT TTT CTG GAT CAG TAG TAT AGC ACC AGG GCC CCT GCGGAT CGT TGT CTG GA-3';
[0056] K1-...
Embodiment 2
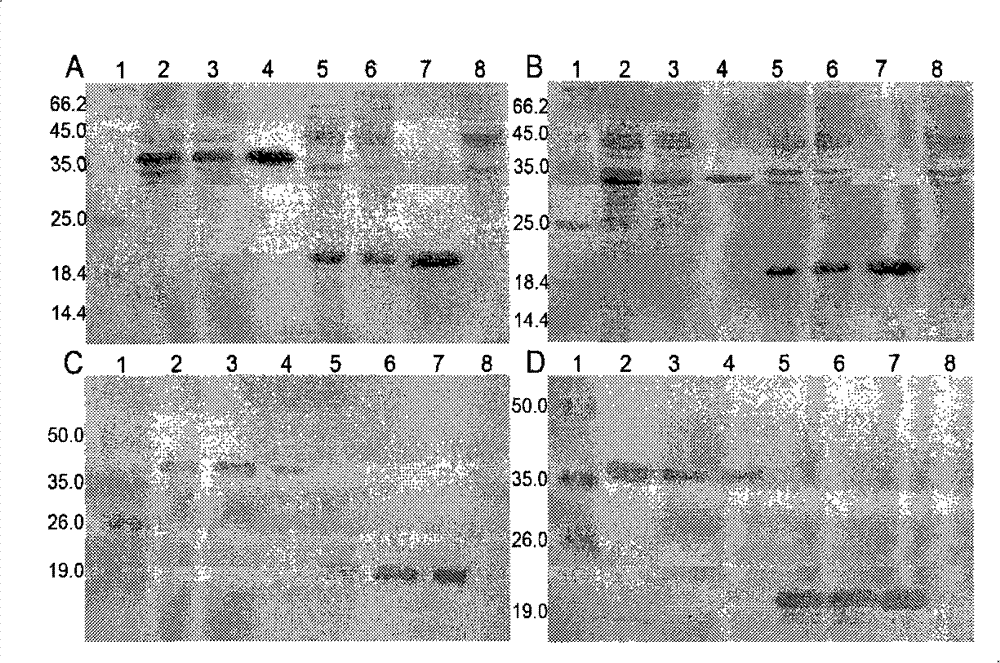
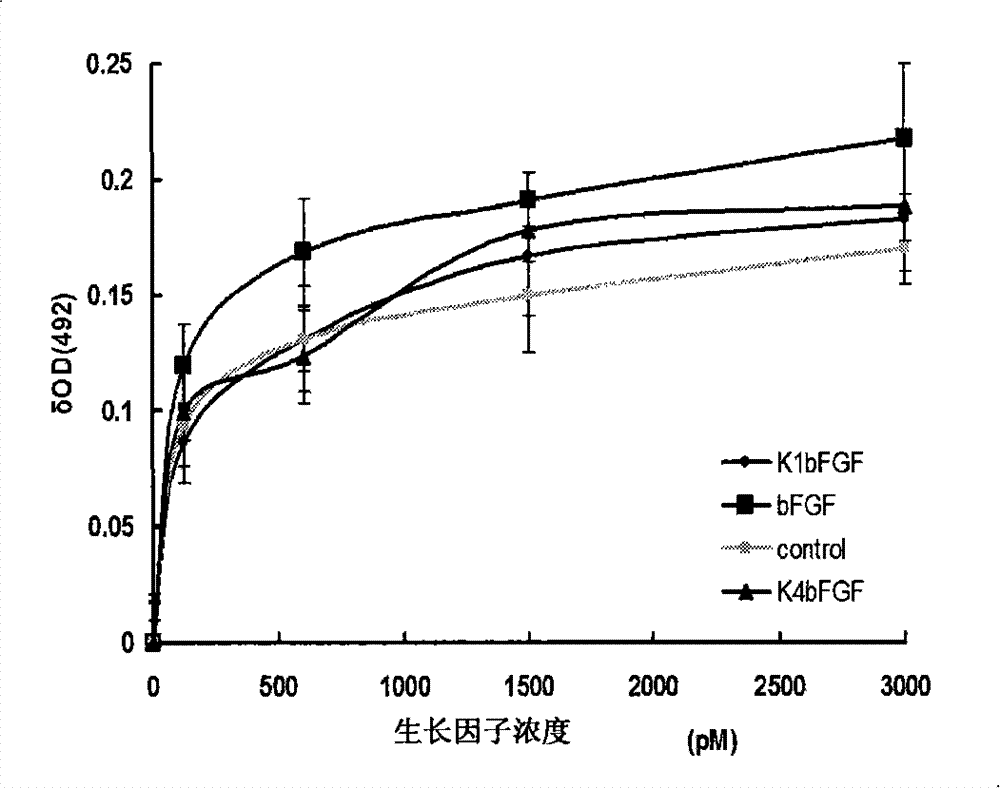
[0075] Example 2, in vitro verification of the biological activity of K1bFGF and K4bFGF
[0076] 1) Identification of mitogen activity of recombinant protein
[0077] Human fibroblasts were used to test the mitogenic activity of the recombinant proteins K1bFGF and K4bFGF.
[0078] Human fibroblasts were inoculated into a 48-well plate at 3000 cells / well, with 10% (volume ratio) fetal bovine serum (FBS), 1% (mass ratio) glutamine, 1% (mass ratio) non-essential amino acid , 100U penicillin / ml and 100μg streptomycin / ml high glucose DMEM medium (H-DMEM), at 37°C, 5% CO 2 and cultured at saturated humidity for 24 hours. After 24 hours, the FBS concentration of the culture medium was changed to 2% (volume ratio), and other conditions remained unchanged. Then add K1bFGF or K4bFGF with final concentrations of 0, 30, 120, 600, 1500 and 3000 pmol / L to each culture well in turn, set 4 parallel samples for each concentration, continue to cultivate for 3 days, and add The final concent...
Embodiment 3
[0102] Example 3, Functional Evaluation of Recombinant Proteins in Animals
[0103] 1) Effect evaluation of K1bFGF and K4bFG in rat skin random ischemia model
[0104] The model of random skin flap in rats can be used to detect angiogenesis. Due to the formation of plasma clots in this model, the recombinant protein can be directly applied to this model to observe the condition of angiogenesis.
[0105] The method of applying recombinant protein to rat skin random ischemia model is as follows:
[0106] 1) Animal model: Rat "random pattern skin flap", male Wistar rats, about 180g, were anesthetized by intraperitoneal injection (i.p.) of pentobarbital sodium at a dose of 40mg / kg body weight; Hair, disinfected with alcohol; cut 4 pieces of 15*15mm on the back 2 The side-based skin cover;
[0107] 2) Apply K1bFGF and bFGF in amounts of 580 pmol (approximately equivalent to 10 μg of natural bFGF) to the wound surface, respectively. The location of each sample was varied in diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com