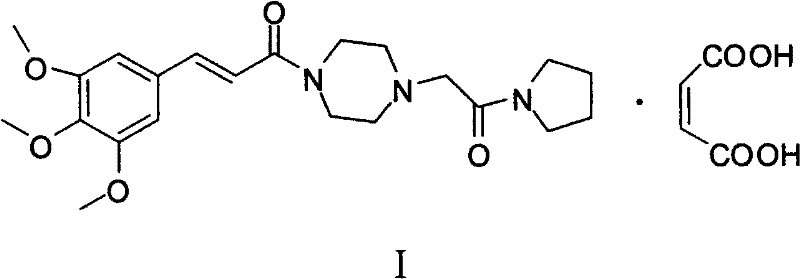
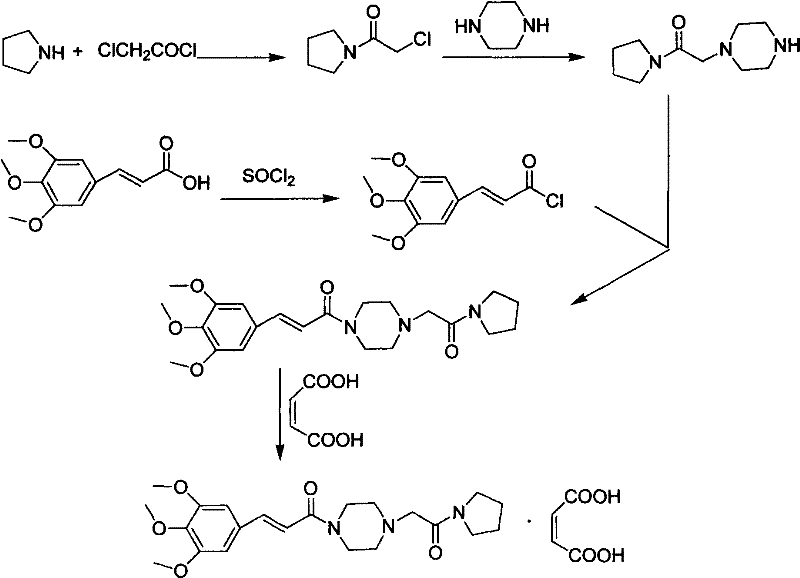
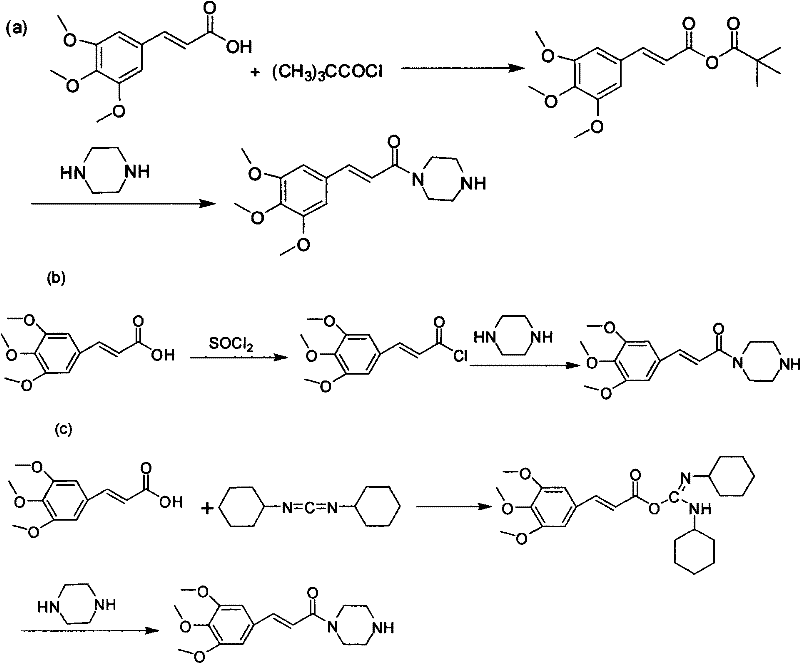
Method for preparing cinepazide maleate
A technology for cinepazide maleate and piperazine, which is applied in the field of chemistry, can solve the problems of difficult removal of double-alkylated impurities, low yield of mono-alkylated products, etc., and achieves the advantages of low cost, simple synthesis method and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of 1-(3,4,5-trimethoxycinnamoyl)piperazine
[0029] Put 83.3g (0.35mol) of 3,4,5-trimethoxycinnamic acid and 700mL of dry dichloromethane in a 2000mL three-neck flask, stir and dissolve at room temperature; mol) triethylamine were diluted with 150mL of dry dichloromethane respectively, and dropped into the above-mentioned reaction flask at the same time. After dropping, react at room temperature for 6h.
[0030] Put 86g (1.0mol) of anhydrous piperazine and 200mL of absolute ethanol in a 2000mL three-neck bottle, stir mechanically until the solution is transparent, and under stirring, drop the above reaction solution into the bottle at room temperature; after dropping, react at room temperature for 2h. The solvent was evaporated to dryness, and 10% dilute hydrochloric acid was slowly added to the residue to pH 1-2, stirred for 30 min, filtered with suction, and the filtrate was adjusted to pH 9-12 with 30% sodium hydroxide solution. Extract with (300 mL...
Embodiment 2
[0036](1) Preparation of 1-(3,4,5-trimethoxycinnamoyl)piperazine
[0037] Put 119g (0.5mol) of 3,4,5-trimethoxycinnamic acid, 72mL (0.6mol) of pivaloyl chloride, 103.5g (0.75mol) of anhydrous potassium carbonate, and 800mL of dry chloroform into a 2000mL three-necked flask , Reaction at room temperature for 6h. Dissolve 172g (2.0mol) of anhydrous piperazine in 300mL of chloroform, stir mechanically until the solution is transparent, add to the above reaction solution, and react at room temperature for 2h.
[0038] Evaporate the solvent to dryness, slowly add 10% dilute hydrochloric acid to pH 1-2, stir for 30 min, filter with suction, and adjust the pH of the filtrate to 9-12 with 30% sodium hydroxide solution. Extract with (300 mL×3) chloroform, wash the organic layer with saturated brine, and dry the organic layer over anhydrous sodium sulfate overnight. Anhydrous sodium sulfate was filtered off, and chloroform was recovered to obtain 146.2 g of yellow oil, yield: 93.7%. ...
Embodiment 3
[0044] (1) Preparation of 1-(3,4,5-trimethoxycinnamoyl)piperazine
[0045] Put 42.8g (0.18mol) of 3,4,5-trimethoxycinnamic acid and 350mL of dry dichloromethane in a 1000mL three-necked bottle, stir and dissolve at room temperature. Dilute 25.8mL (0.21mol) of pivaloyl chloride and 26.5g (0.25mol) of triethylamine with 80mL of dry dichloromethane, respectively, and drop them into the above reaction flask at the same time; after dropping, heat to reflux for 2h.
[0046] 43g (0.5mol) of anhydrous piperazine was dissolved in 100mL of absolute ethanol, added to the above reaction solution, and reacted at room temperature for 1 hour. Evaporate the solvent to dryness, adjust the pH value to 1-2 with 10% dilute hydrochloric acid, filter with suction, and adjust the pH value of the filtrate to about 9-12 with 30% sodium hydroxide solution. Extract with (200mL×3) dichloromethane, wash the organic layer with saturated brine, and dry over anhydrous sodium sulfate; filter with suction, re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com