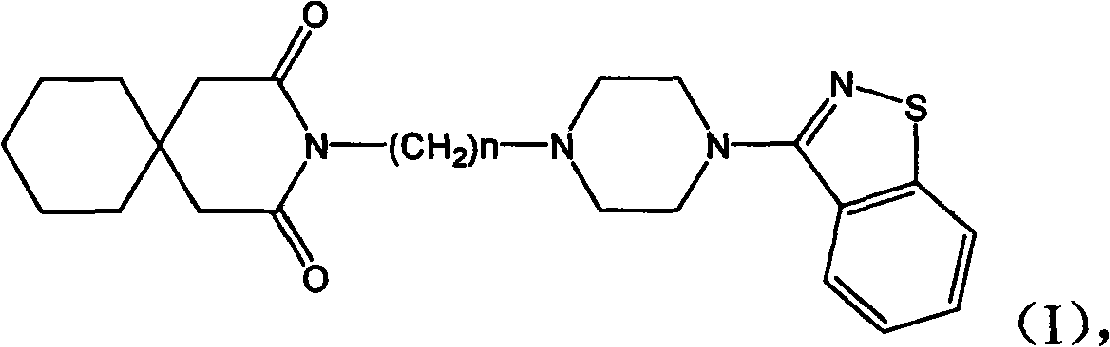
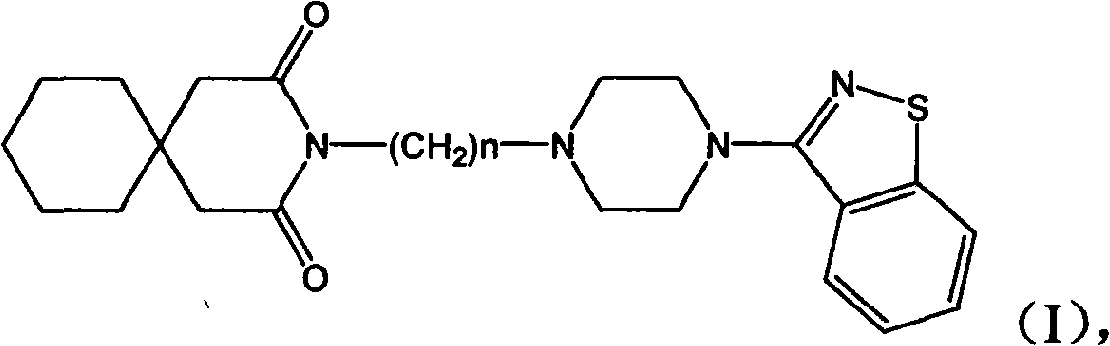
N-aryl piperazine derivative and preparation method thereof and drug composition adopting N-aryl piperazine derivative as active ingredient
A technology of arylpiperazine and derivatives, applied in the field of N-arylpiperazine derivatives, can solve problems such as adverse reactions and lack of selectivity in action, and achieve the effect of reducing side effects and improving negative symptoms and positive symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] N-[3-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]propyl]-3,3-cyclopentane glutarimide hydrochloride
[0018]
[0019] 3,3-cyclopentane glutarimide 0.9g (5mmol), 1-bromo-3-chloropropane 0.79g (5mmol), potassium carbonate 1.5g (11mmol) in 10ml DMF (dimethylformamide) 90 Stir at ℃ for 2 h, add 0.87 g (4 mmol) of 1-(1,2-benzisothiazol-3-yl) piperazine and continue to stir at this temperature for 5 h, let the reaction cool down, pour into water, extract with ethyl acetate, The organic layer was washed with water, washed with brine, dried over magnesium sulfate, concentrated to dryness, dissolved in isopropanol, added HCl-EtOH (hydrochloric acid-ethanol) solution to pH=3, a solid precipitated, filtered to obtain 0.7 g of a white solid, mp( Melting point): 250°C, ESI-MS (m / z): 440.54 (M+H).
Embodiment 2
[0021] N-[4-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]butyl]-3,3-cyclopentaneglutarimide hydrochloride
[0022]
[0023] 0.8g (4.4mmol) of 3,3-cyclopentane glutarimide, 0.7g (4.4mmol) of 1-bromo-4-chlorobutane, and 1.4g (10.3mmol) of potassium carbonate were stirred in 10ml DMF at 90°C for 2h , add 0.8g (3.7mmol) of 1-(1,2-benzisothiazol-3-yl)piperazine and continue to stir at this temperature for 5h. After the reaction is left to cool, it is washed into water, extracted with ethyl acetate, and the organic layer is Washed with water, washed with brine, dried over magnesium sulfate, concentrated to dryness, dissolved in isopropanol, added HCl-EtOH solution to pH = 3, precipitated solid, filtered to obtain 0.6g of white solid, mp: 200-204°C, ESI- MS (m / z): 454.56 (M+H).
Embodiment 3
[0025] N-[2-[4-(1,2-Benzisothiazol-3-yl)-1-piperazinyl]ethyl]-3,3-cyclopentaneglutarimide
[0026]
[0027] 3,3-cyclopentane glutarimide 1g (5.6mmol), 1-bromo-2-chloroethane 0.9g (5.7mmol), potassium carbonate 1.6g (11.7mmol) in 10mlDMF (dimethylformamide ) at 90°C for 2 h, add 1 g (4.6 mmol) of 1-(1,2-benzisothiazol-3-yl) piperazine and continue to stir at this temperature for 5 h, let the reaction cool down and pour into water to precipitate a solid. 0.8 g of white solid was obtained by filtration, mp (melting point): 280° C., ESI-MS (m / z): 431.52 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com