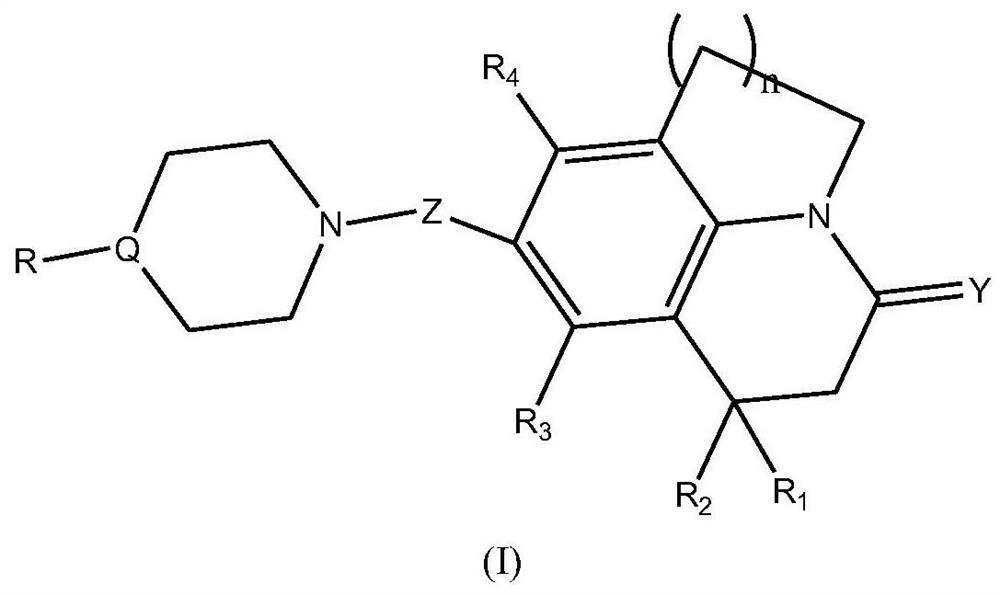
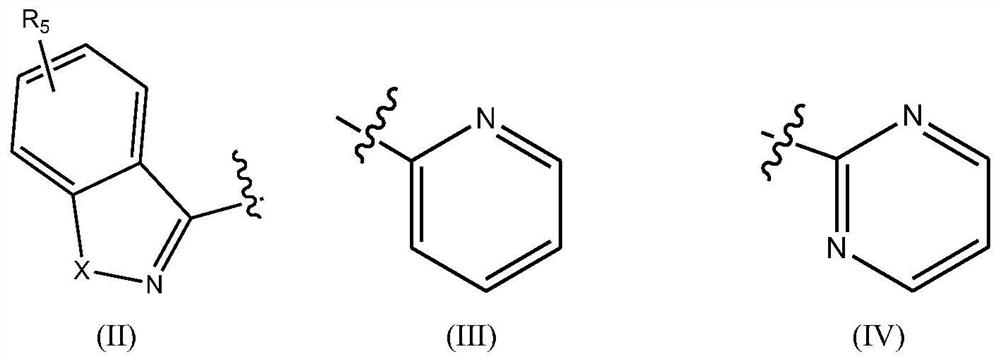
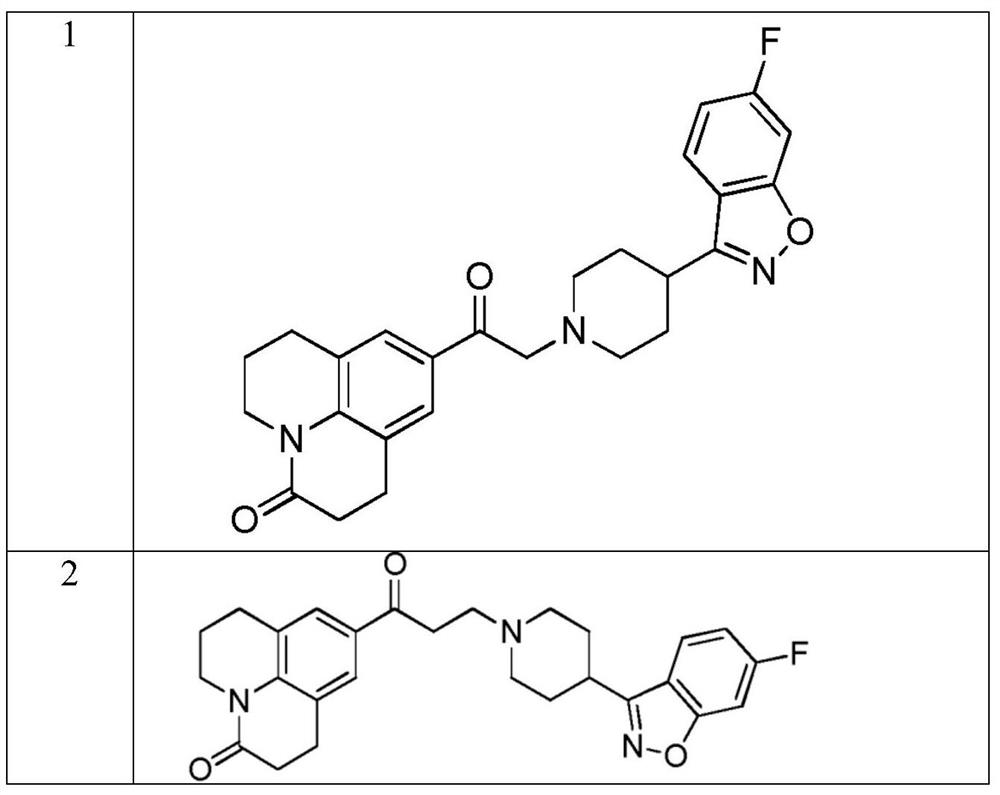
Fused heterocyclic compound derivatives and their applications
A compound and drug technology, applied in the field of condensed heterocyclic compound derivatives, synthesis of condensed heterocyclic compound derivatives, preparation of drugs for the prevention or treatment of neuropsychiatric diseases, can solve problems such as QT gap prolongation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080]
[0081] 1) Take 1,2,3,4-tetrahydroquinoline (5.0g), add acetone (50ml), then slowly add 3-chloropropionyl chloride (5.1g), stir until reflux, and the reaction is complete after 4 hours. The reaction mixture was cooled to room temperature, concentrated, dissolved in ethyl acetate, washed with water, dried over anhydrous magnesium sulfate, filtered with suction, and concentrated to obtain 8.0 g of an oil. Yield 96.4%.
[0082] 2) Take the product (8 g) of the first step, add anhydrous aluminum trichloride (7.2 g), and react under stirring for 3 hours. After cooling the reaction mixture to room temperature, ice water was added to quench the system. The reaction mixture was filtered, extracted with ethyl acetate, washed with water, dried over anhydrous magnesium sulfate, filtered with suction, and concentrated to obtain 6.05 g of solid. Yield 90.3%.
[0083]3) Take the product of the second step (5.0 g), add 2-chloroacetyl chloride (2.30 mL) and 1,2-dichloroethane (2...
Embodiment 2
[0087] The title compound was prepared according to the method of Example 1, but 3-chloropropionyl chloride was used instead of 2-chloroacetyl chloride.
[0088] 1H NMR (600MHz, CDCl3) δ7.76(s,1H),7.72(s,1H),7.21–7.11(m,2H),7.03–6.86(m,1H),3.93(dt,J=12.9,6.4 Hz,4H),3.42(t,J=6.8Hz,2H),3.20(t,J=7.3Hz,2H),3.11(s,4H),3.05–2.89(m,2H),2.87(d,J =6.2Hz,2H),2.79–2.65(m,2H),2.05–1.92(m,3H).
[0089] MS(ESI)m / z462.5([M+H]+)
Embodiment 3
[0091]
[0092] The target compound (0.5 g) of Example 2 was dissolved in anhydrous methanol (20 mL), and the reaction mixture was cooled to zero with an ice-water bath, sodium borohydride (0.08 g) was added thereto, and stirring was continued for 1 h. The reaction was quenched by adding 10 mL of water. The solvent was evaporated to dryness, an appropriate amount of dichloromethane was added, washed with water, and the aqueous layer was separated. The organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated to obtain a pale yellow oil. Column chromatography gave 0.40 g of white solid.
[0093] 1H NMR (600MHz, CDCl3) δ7.71(dt, J=11.6,5.8Hz,1H),7.68(s,1H),7.66(s,1H),7.26(dd,J=8.5,2.0Hz,1H) ,7.12–7.02(m,1H),3.94–3.88(m,2H),3.20(t,J=7.3Hz,2H),3.10(dd,J=15.3,7.3Hz,3H),3.00–2.95(m ,2H),2.92(t,J=7.3Hz,2H),2.87(t,J=6.2Hz,2H),2.73–2.69(m,2H),2.30(t,J=12.5Hz,2H),2.14 –2.05(m,4H),1.99(dt,J=12.1,6.1Hz,2H),1.86-1.80(m,2H).MS(ESI)m / z464.7([M+H] + )
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com