Terpolymer containing polyester chain links and polycarbonate chain links and synthetic method thereof
A terpolymer and a synthesis method technology are applied to the terpolymer containing polyester chain segment and polycarbonate chain segment and the field of synthesis thereof, which can solve the problem that the degradability of the polycarbonate segment is not improved, and the cyclic cyclone cannot be avoided. Carbonate, low catalyst activity, etc., to achieve the effect of inhibiting self-polymerization and inhibiting the generation of polyether chain units
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthesis of embodiment 1 polyester
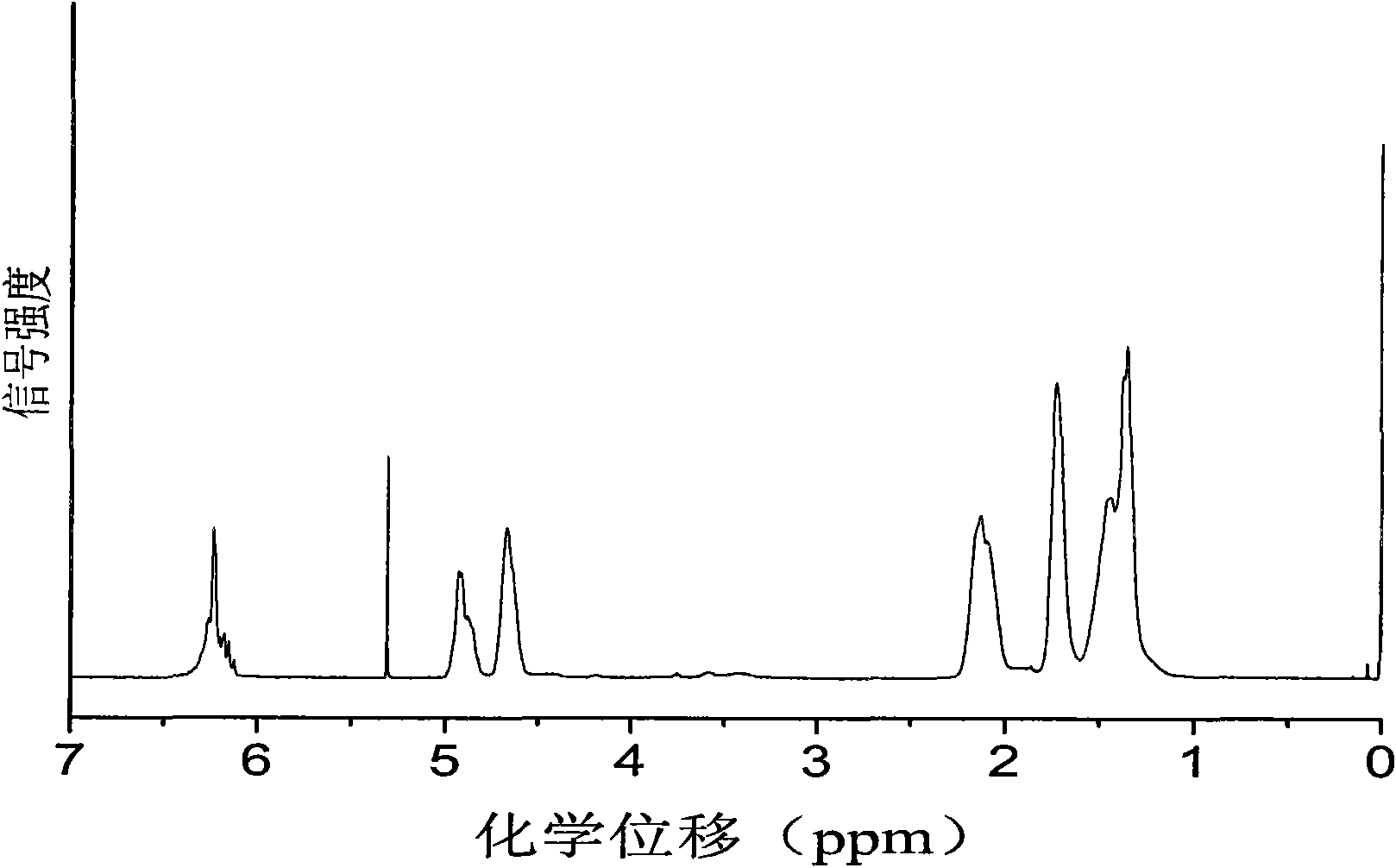
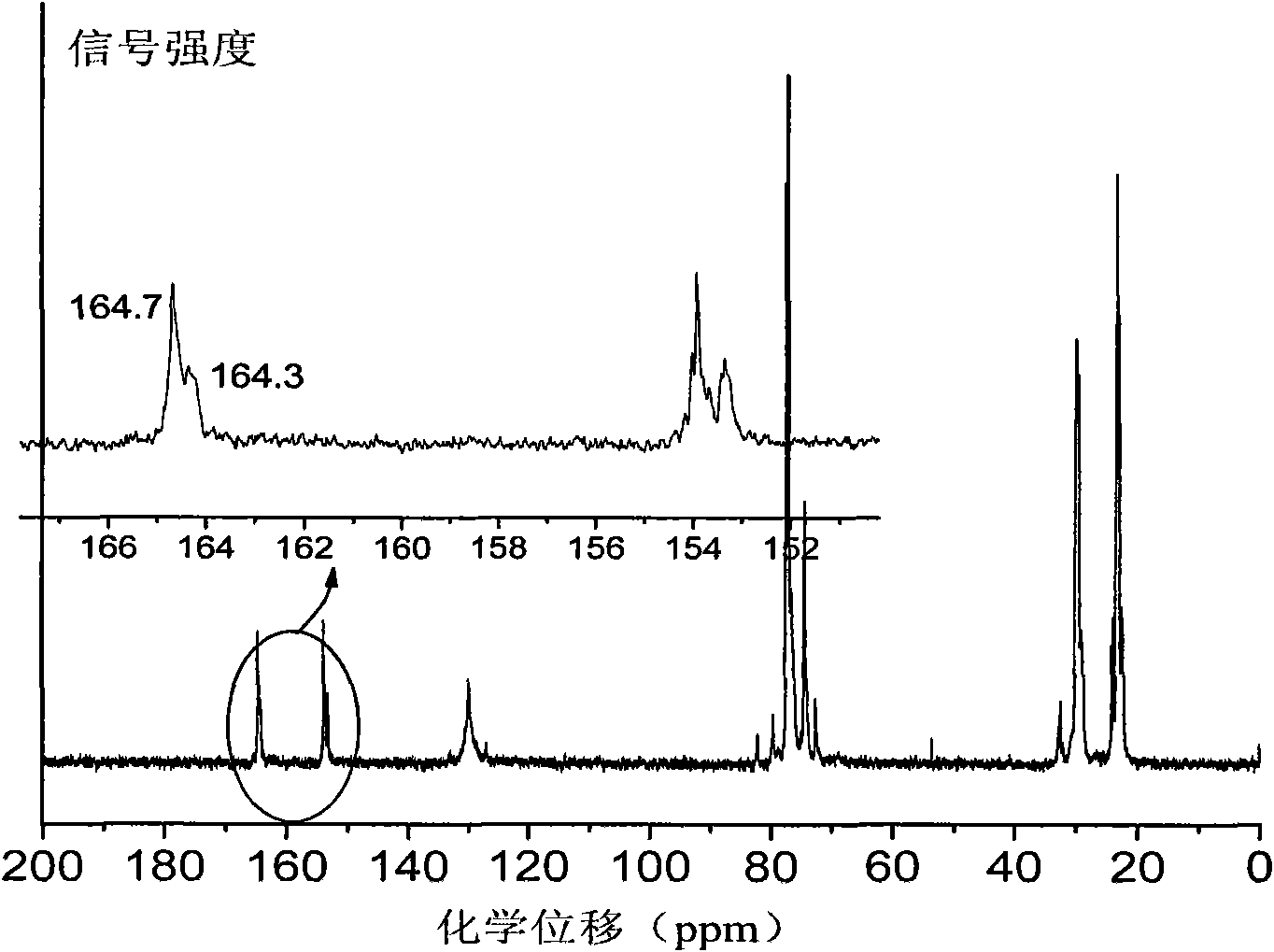
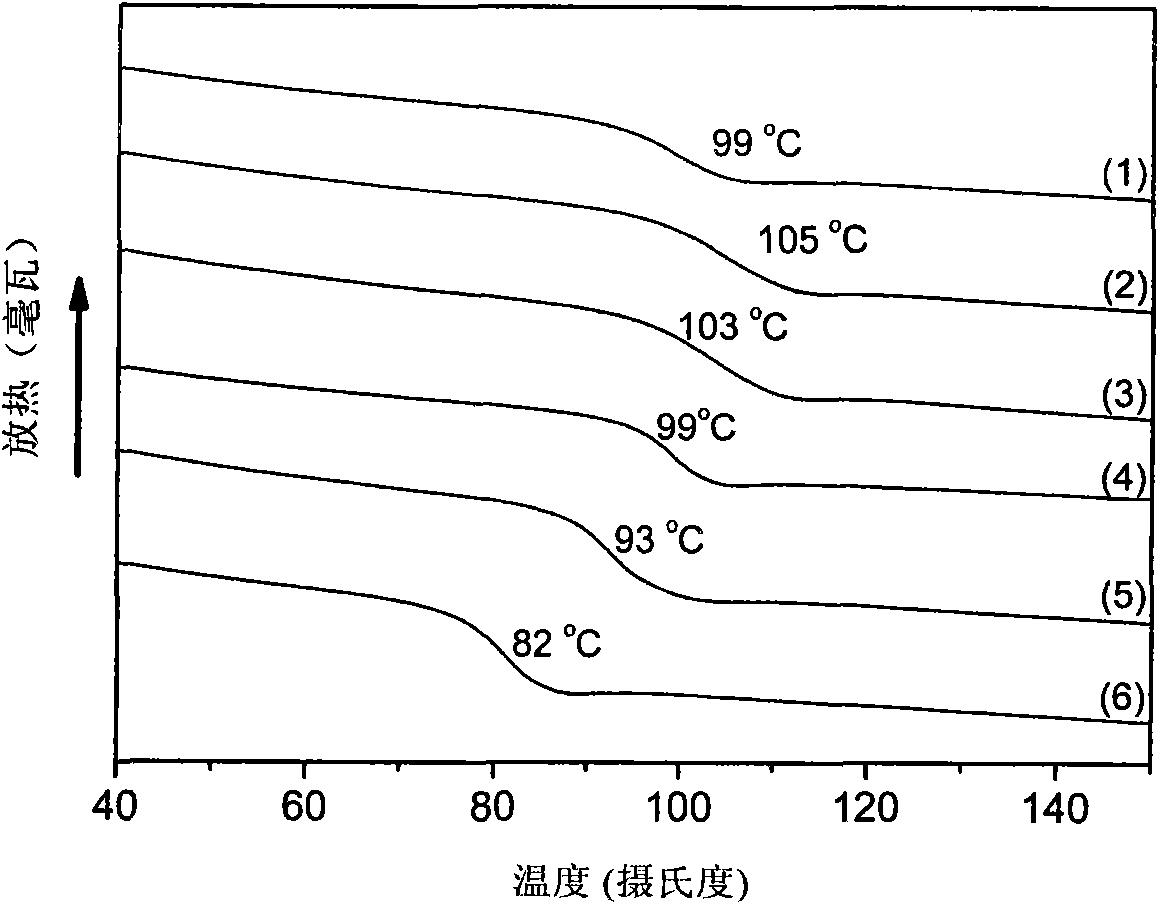
[0039] Before the polymerization reaction, the high-pressure reaction kettle with a capacity of 60ml was removed at 110°C for about 2 hours and cooled to room temperature in a drying tower. Add 3.0mg of Zn-Co DMC, 2.0g of maleic anhydride, 4.0mL of cyclohexene oxide and 4.0mL of tetrahydrofuran to the reaction kettle in sequence, seal the reaction kettle, add it to an oil bath preheated to 90°C, and stir the reaction with magnetic force After 5 hours, the temperature was rapidly lowered, the pressure was released, and the crude product was taken out. First remove the solvent under reduced pressure, then dissolve the crude product with THF, precipitate the polymer in methanol, and obtain a yellow product after vacuum drying. The conversion rate is calculated by weighing method, and the content of polyester chain and polyether chain is calculated by nuclear magnetic hydrogen spectroscopy. . The test results are shown in Table 2....
Embodiment 2
[0040] The synthesis of embodiment 2 polycarbonates
[0041] Before the polymerization reaction, the high-pressure reaction kettle with a capacity of 60ml was removed at 110°C for about 2 hours and cooled to room temperature in a drying tower. Add 3.0mg of Zn-Co DMC, 4.0mL of cyclohexene oxide, and 4.0mL of tetrahydrofuran in sequence, seal the reaction kettle, add it to an oil bath preheated to 90°C, stir it magnetically, and then fill it with CO 2 to CO 2 The pressure was 4.0 MPa. After reacting for 5 hours, the temperature was rapidly lowered, the pressure was released, and the crude product was taken out. The crude product was subjected to CH 2 Cl 2 After washing with / methanol and drying in vacuum, a yellow product was obtained, the conversion rate was calculated by weighing method, and the content of polyester chain unit and polyether chain unit was calculated by hydrogen nuclear magnetic spectrum. The test results are shown in Table 2.
Embodiment 3
[0042] The synthesis of embodiment 3 polycarbonates
[0043] The method of Example 2 was adopted, the only difference being that dioxane was used instead of tetrahydrofuran as a solvent. The test results are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com