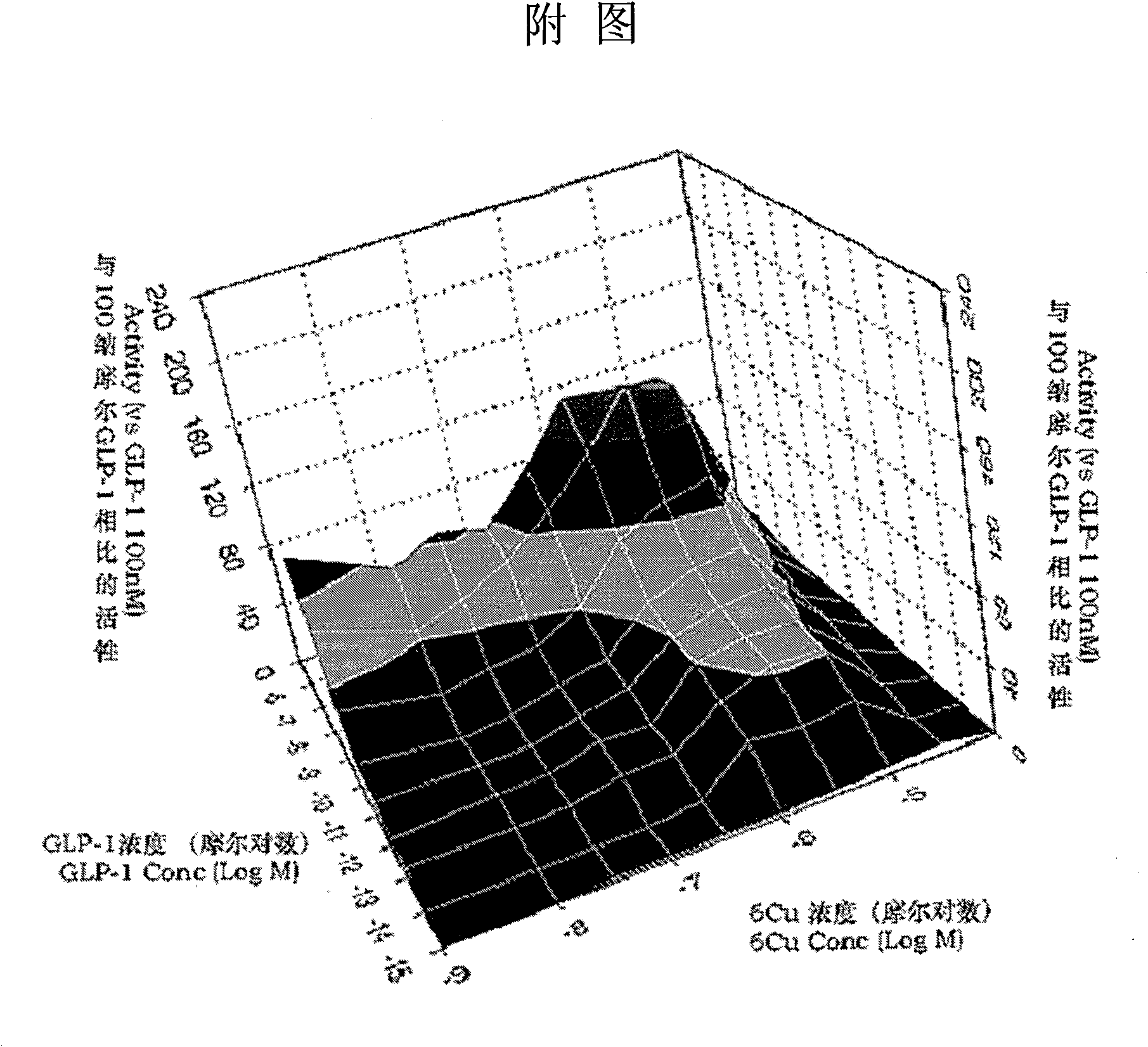
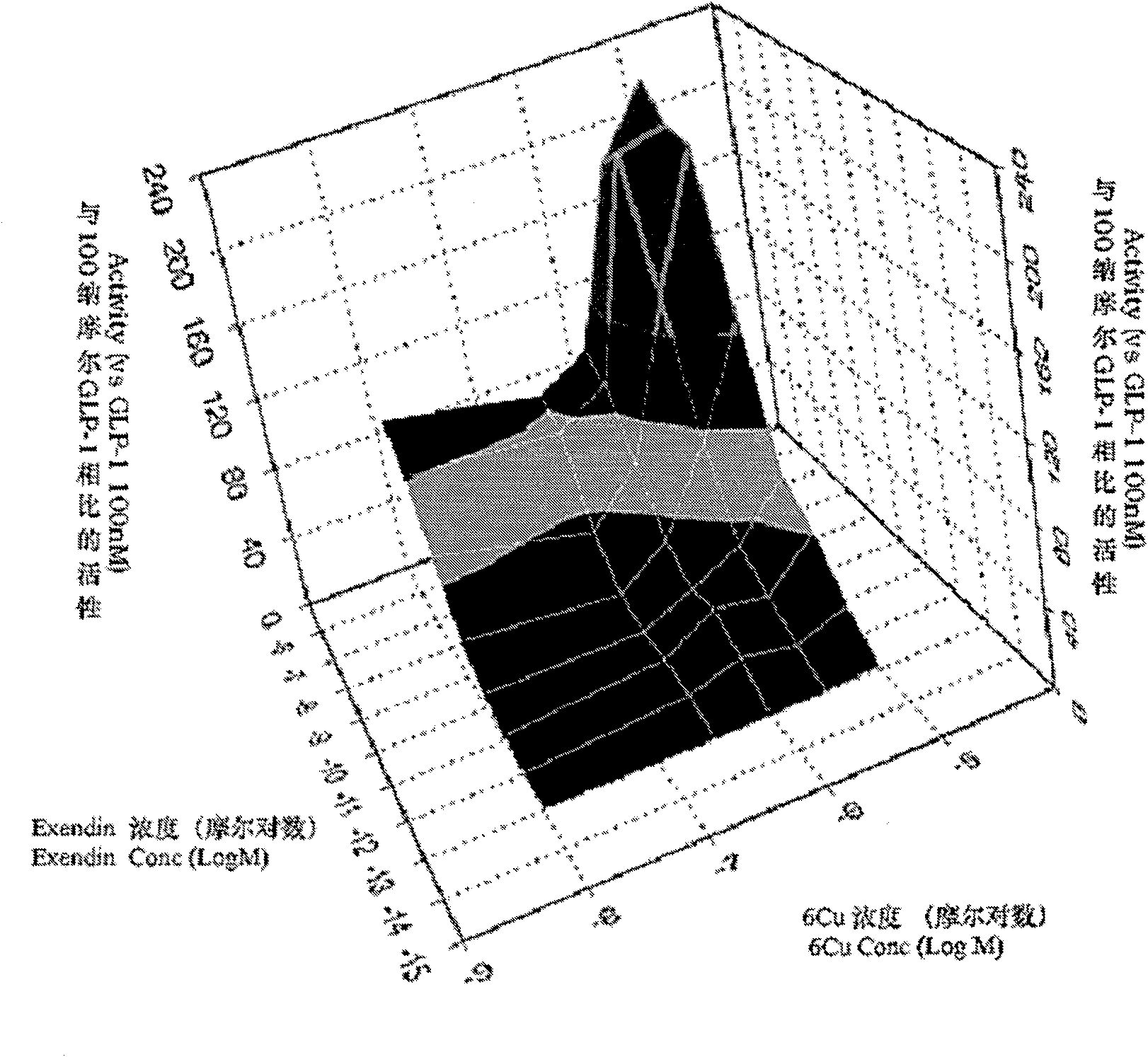
Receptor signal transduction positive modulator, preparation method and purpose thereof
A technology of solvates and compounds, which can be used in medical preparations containing active ingredients, pharmaceutical formulations, metabolic diseases, etc., and can solve the problem of inconvenient oral administration of polypeptide drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Embodiment 1. Preparation of compound 6Aa
[0140] [Chemical Reaction Formula 5]
[0141]
[0142] According to the chemical reaction above:
[0143] Step 1: Add 1.46g (0.01mol) CH to a 100mL three-necked bottle 3 NCS, 2.5g (0.0098mol) of p-chloroaniline and 20mL of absolute ethanol were stirred until the solid was dissolved, heated to reflux, and followed by TLC to monitor the reaction. After the reaction was completed, the reaction solution was placed in a refrigerator at 4° C. for cooling and crystallization, filtered with suction, and dried to obtain 3.4 g of white solid 3a (yield 87%).
[0144] Step 2: Add 3.4g (0.0169mol) of compound 3a, 4.8g (0.0508mol) of chloroacetic acid, 4.17g (0.0508mol) of sodium acetate, a small amount of TEBA and 20mL of absolute ethanol into a 100mL three-necked flask. Heated to reflux, followed by TLC to monitor the reaction. After the reaction was completed, it was filtered while it was hot, and the filtrate was concentrated und...
Embodiment 2
[0147] The preparation of embodiment 2. compound 6Ba
[0148] [Chemical Reaction Formula 6]
[0149]
[0150] According to the chemical reaction above:
[0151] Step 1: Compound 3b was prepared in the same manner as the first step in Example 1, except that raw materials 1b and 2b were used instead of 1a and 2a in the first step in Example 1, respectively.
[0152] The second step: Compound 4b was prepared in the same manner as the second step in Example 1, except that 3a in the second step in Example 1 was replaced by starting material 3b.
[0153] The third step: Compound 6Ba was prepared by the same method as the third step in Example 1, except that raw materials 4b and 5b were used instead of 4a and 5a in the third step in Example 1, respectively.
[0154] Compound 6Ba: 1 H NMR (300MHz, DMSO-d 6 )δ0.930(t, 3H, J=7.35Hz), 1.358(sex, 2H, J=7.5Hz), 1.691(qu, 2H, J=7.35Hz), 2.283(s, 6H), 3.892(t, 2H, J=6.9Hz), 7.055(d, 2H, J=8.4Hz), 7.393(d, 1H, J=8.4Hz), 7.457(m, 4H), ...
Embodiment 3
[0155] Embodiment 3. Preparation of compound 6Ca
[0156] [Chemical Reaction Formula 7]
[0157]
[0158] According to the chemical reaction above:
[0159] Step 1: Add 1.7g (22mmol) NH to a 50mL three-necked bottle 4 SCN and 10mL of acetone were stirred at room temperature until the solid was dissolved, and 2.82g (20mmol) of benzoyl chloride was slowly added dropwise, a white solid appeared, and the solution gradually turned yellow and thickened. After the dropwise addition, reflux for half an hour, and slowly add 2.551 g (20 mmol) of p-chloroaniline 2a in acetone (10 mL) dropwise to the reaction system. After the dropwise addition, the reflux was continued, and the reaction was tracked and monitored by TLC. After the reaction was completed, it was cooled, and the reaction solution was poured into 150 mL of water, stirred, and a yellow solid was precipitated. After suction filtration, the solid was dissolved in 3 g NaOH aqueous solution (27 mL), boiled for 5 minutes, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com