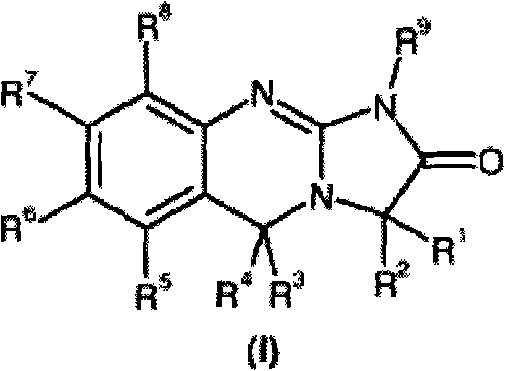
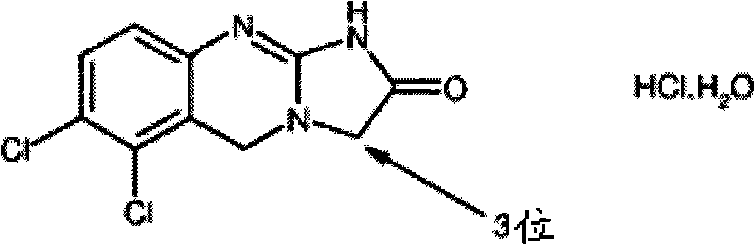
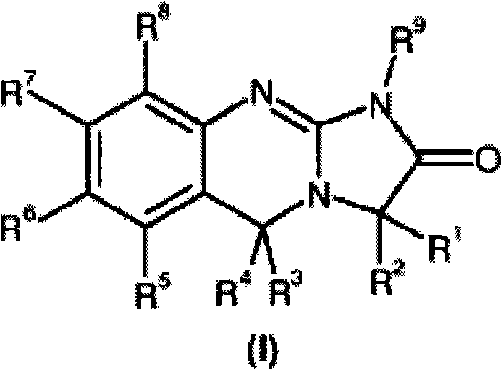
Substituted quinazolines
A technology of metabolic reactions, compounds, applied in extracellular fluid diseases, drug combinations, organic chemistry, etc., can solve the problem of unawareness of anti-megakaryocyte potential and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] IC of anagrelide and certain 3-alkyl substituted analogues as PDE III inhibitors and antimegakaryocyte agents 50 Comparative data.
[0154] The table below shows the comparative activity of anagrelide and its analogs with respect to their effects on megakaryopoiesis (the process of producing platelets) and PDE III (the inhibition of which leads to adverse cardiovascular reactions).
[0155] Comparison of In Vitro Evaluation of Potential Therapeutic Effects and Adverse Effects of Anagrelide and Its Analogues
[0156] compound
IC against megakaryocytes 50
(thrombocytopenia activity)
IC for PDE III inhibition 50
(cardiovascular effects)
Benefit rate (therapeutic effect
compared with adverse effects)
Anagra
3-Hydroxyanagrelide
27nM
44nM
32nM
0.7nM
*0.024∶1
0.016∶1
3,3-Dimethyl anagrelide
164nM
166nM
1∶1
3-Spirocyclopropyl anagrelide
547nM
797nM
1.45∶1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com