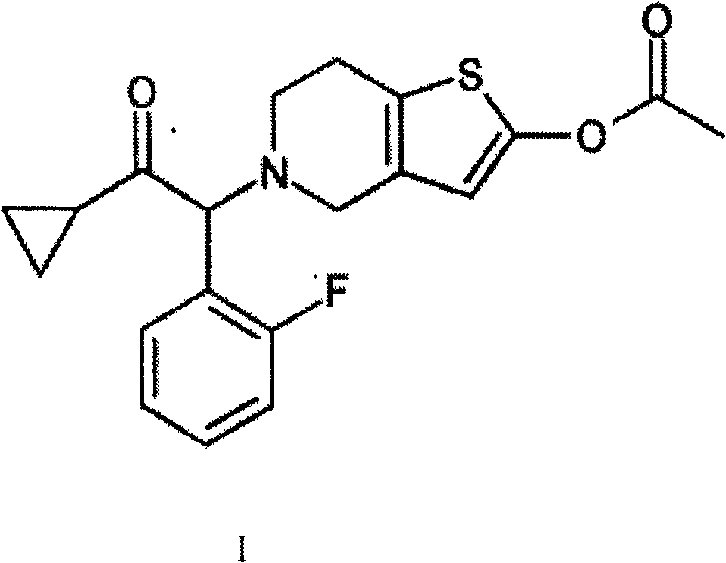
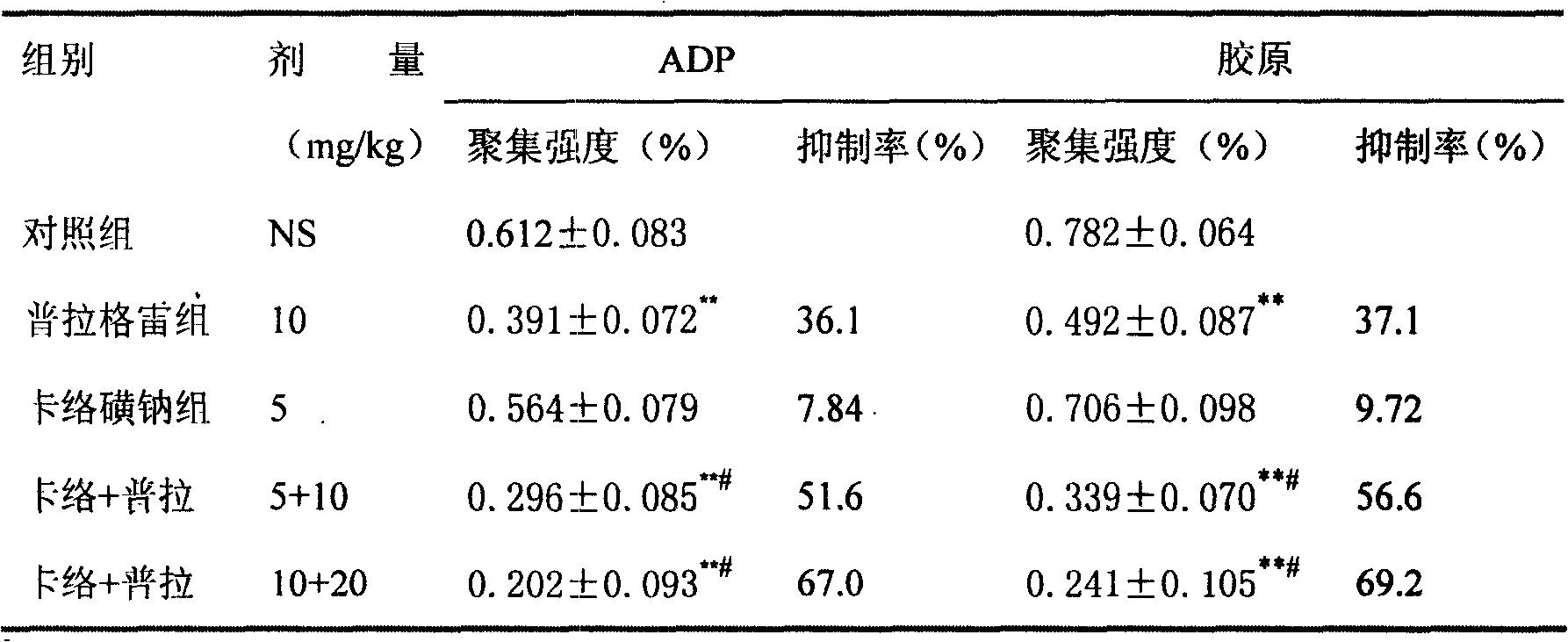
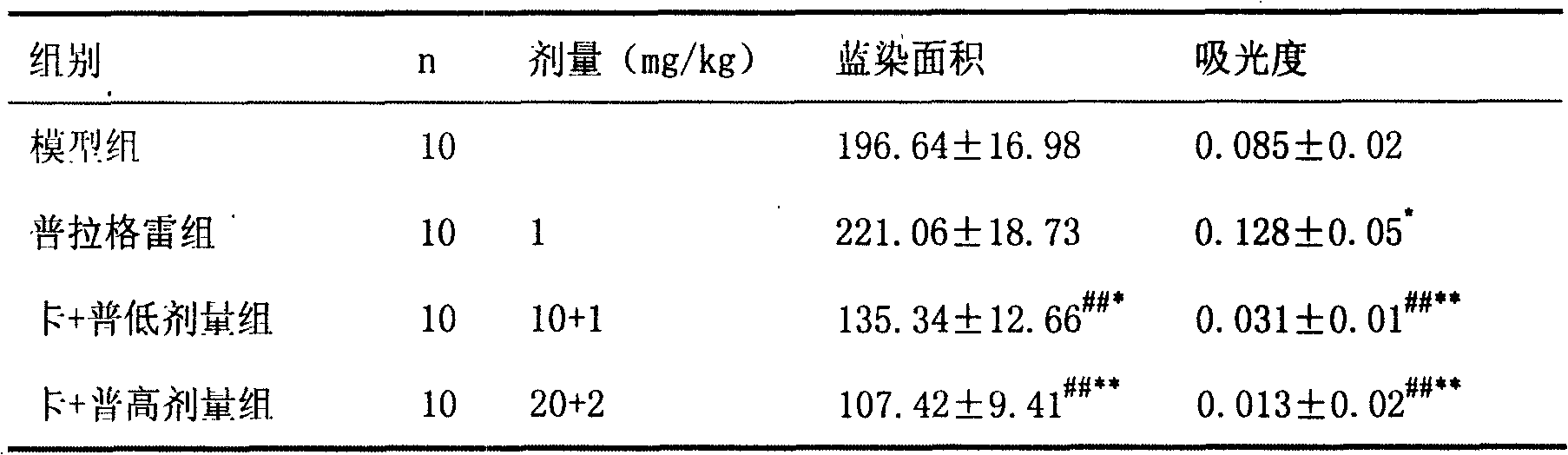
Pharmaceutical composition containing prasugrel and carbazochrome sodium sulfonate
A technology of sodium carbosulfonate and its composition, which is applied in the field of new pharmaceutical compositions, can solve problems such as reducing patient compliance and rebounding of cardiovascular events, and achieve the effect of inhibiting platelet aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1 common tablet
[0015] Sodium carbosulfonate 50g
[0016] Prasugrel Maleate 5g
[0017] Microcrystalline Cellulose 250g
[0018] Lactose 20g
[0019] 10% starch slurry appropriate amount
[0020] Magnesium stearate 0.8g
[0021] Preparation process: Weigh the prescribed amount of sodium carbosulfonate, prasugrel maleate, microcrystalline cellulose, and lactose and mix evenly. In addition, add an appropriate amount of 10% starch slurry to the mixed powder, mix evenly, make soft materials, pass through a 18-mesh nylon sieve to make wet granules, and dry at about 60°C. The moisture content of dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
Embodiment 2
[0022] Embodiment 2 common tablet
[0023] Sodium carbosulfonate 10g
[0024] Prasugrel HCl 20g
[0025] Starch 140g
[0026] Dextrin 120g
[0027] 50% ethanol appropriate amount
[0028] Magnesium Stearate 1.0g
[0029] Preparation process: Weigh the prescription amount of sodium carbosulfonate, prasugrel hydrochloride, starch and dextrin and mix evenly. In addition, an appropriate amount of 50% ethanol is added to the mixed powder, mixed evenly, made into soft material, passed through a 18-mesh nylon sieve to make wet granules, dried at about 60°C, and the moisture content of the dry granules should be controlled below 1.5%. Sieve through a 20-mesh sieve, mix with magnesium stearate, and press into tablets.
Embodiment 3
[0030] Embodiment 3 Dispersible tablets
[0031] Sodium carbosulfonate 5g
[0032] Prasugrel 25g
[0033] Croscarmellose Sodium 10g
[0034] Microcrystalline Cellulose 150g
[0035] Polyvinylpyrrolidone 5.5g
[0036] 5% PVP 60% ethanol solution appropriate amount
[0037] Micronized silica gel 5g
[0038]Preparation process: Weigh carbosulfonate sodium and prasugrel according to the prescription, use microcrystalline cellulose as filler, cross-linked carmellose sodium and polyvinylpyrrolidone as disintegrants, 5% PVP 60% alcohol solution It is used as binder, and micronized silica gel is used as flow aid. It is granulated in one step in a fluidized bed, and then compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com