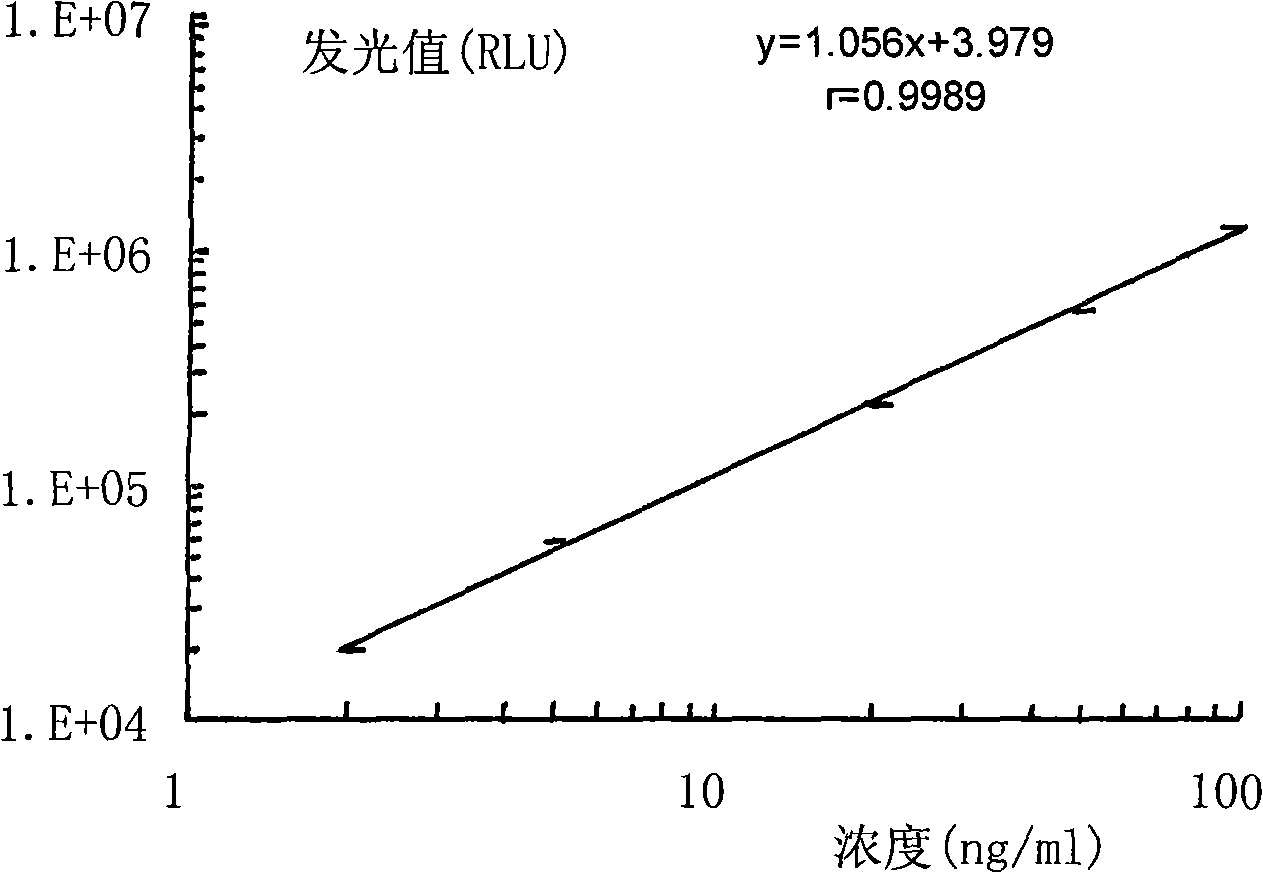
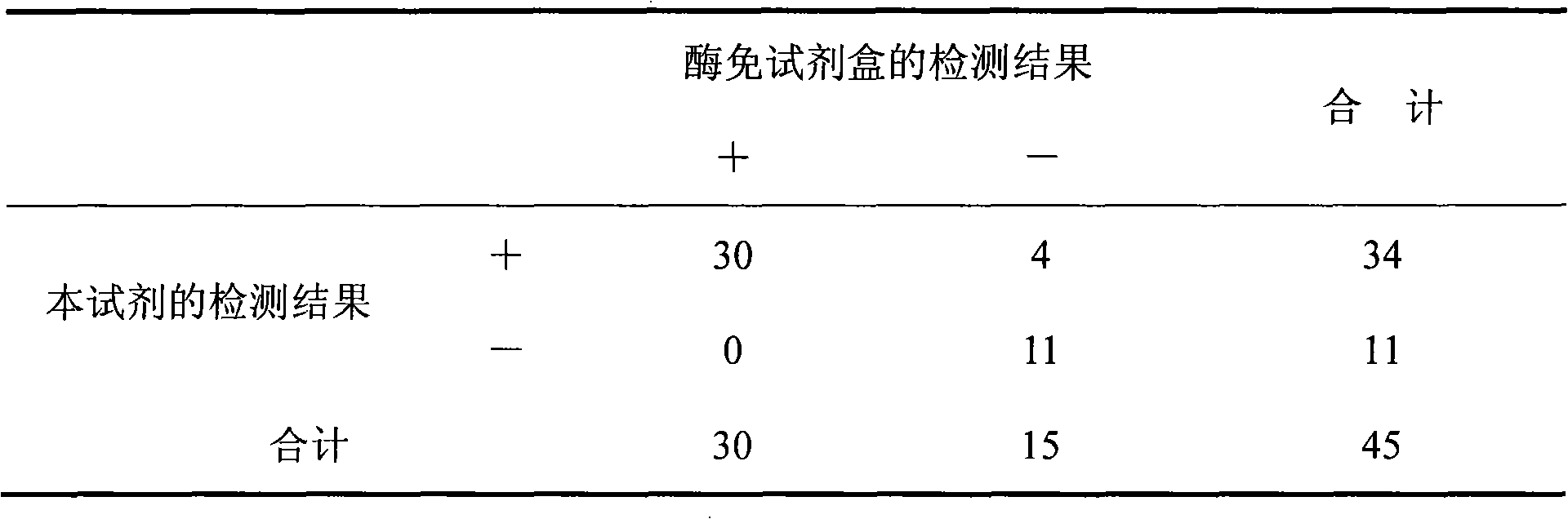
Hepatitis B virus pre S1 antigen chemiluminscence immunoassay kit and preparation method thereof
A technology of hepatitis B virus and reagent kit, which is applied in the field of immunoanalysis medicine, can solve problems such as the inability to widely apply clinical diagnosis and scientific research work, and limit popularization and use, and achieve industrialization and quality control, no cross-reaction, and clinical The effect of improving the matching rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of Hepatitis B Virus Pre-S1 Antigen Chemiluminescence Immunoassay Assay Kit of the present invention
[0035] 1. Preparation of acridinium ester-labeled antibody and biotin-conjugated antibody
[0036] 1. Acridine ester labeling method
[0037] (1) Add 500 μl of 1 mg / ml antibody to be labeled (pH7.20.050M PBS) into a brown glass bottle;
[0038] (2) Add 10 μl of acridinium ester (5 mM DMF solution) into the above-mentioned brown glass bottle, and react with the antibody to be labeled at room temperature in the dark for 30 minutes;
[0039] (3) Add 100 μl of 30 mg / ml lysine solution to terminate the reaction;
[0040] (4) ammonium sulfate precipitation, sephadex column separation, and purification of the acridinium ester-labeled antibody;
[0041] (5) Add the labeled antibody to 1% BSA, 50% glycerol and freeze it.
[0042] 2. Preparation of biotin-conjugated hepatitis B virus pre-S1 monoclonal antibody
[0043] (1) Dissolve biotin (BNHS) in N, N-...
Embodiment 2
[0095] Example 2 Preparation of the hepatitis B virus pre-S1 chemiluminescence immunoassay assay kit of the present invention
[0096] Except for using plastic beads as the solid-phase carrier, the rest were prepared in the same manner as in Example 1 to prepare the hepatitis B virus pre-S1 chemiluminescent immunoassay assay kit.
Embodiment 3
[0097] Example 3 Preparation of Hepatitis B Virus Pre-S1 Chemiluminescence Immunoassay Assay Kit of the present invention
[0098] Except that the magnetic particles were used as the carrier and the magnetic particle-immobilized avidin was prepared by the glutaraldehyde coupling method, the remaining hepatitis B virus pre-S1 chemiluminescent immunoassay assay kits were prepared in the same manner as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com