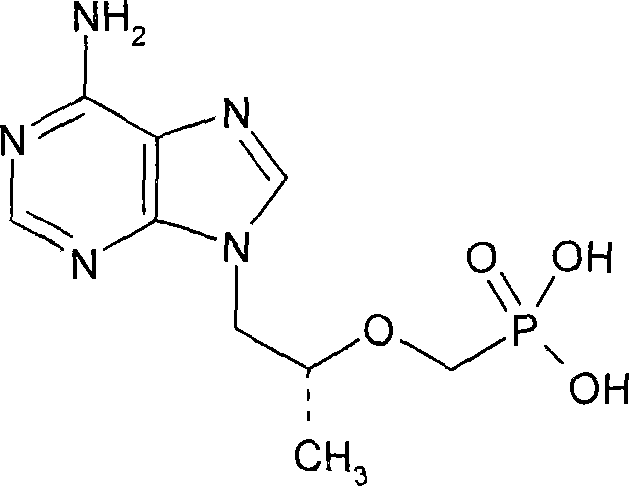
Method for synthesizing (R)-9(2-(diethyl phosphonyl methoxyl) propyl)-adenine
A technology of diethylphosphonomethoxy and phosphonomethoxy, which is applied in the field of synthesizing 9-[2-propyl]-adenine, can solve the difficulties in production operation, high production cost of PMPA diethyl ester, The purity of PMPA diethyl ester is only 40-45%, which achieves the effect of easy control of process operation and safe reagent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (R)-Hydroxypropyladenine
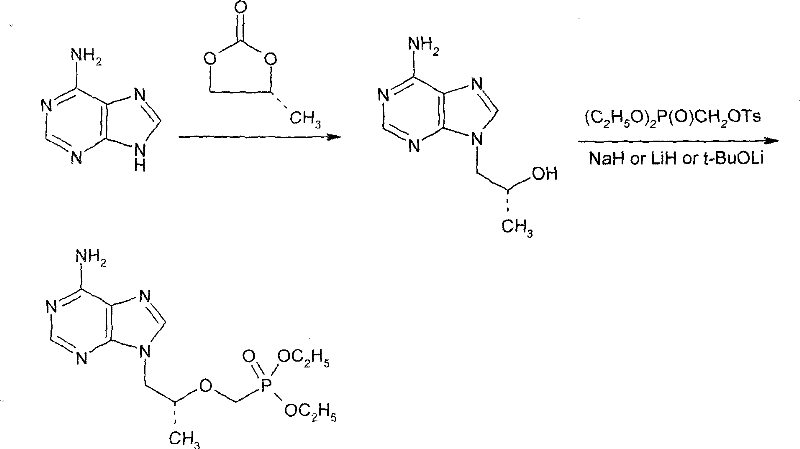
[0021] Under nitrogen protection, put 10.0 g of adenine, 0.12 g of sodium hydroxide, 8.3 g of (R)-propylene carbonate, and 70 ml of DMF into the reaction flask, and heat the mixture in stages: 90°C, 110°C and 125°C, At an interval of 1 hour, the reaction was incubated at an internal temperature of 125°C for 28 hours. After TLC showed the end of the reaction, (R)-hydroxypropyl adenine was obtained and stored at a lower temperature. The purity by area normalized HPLC analysis was 83%.
Embodiment 2
[0023] (R)-9-[2-(Diethylphosphonomethoxy)propyl]-adenine (PMPA diethyl ester)
[0024] Under the protection of nitrogen, in all the solutions obtained in Example 1, 12.0 g of di-n-butyl magnesium was added, and then 38.0 g of p-toluenesulfonyloxymethyl diethyl phosphate was added dropwise (adding in three times, each interval 1 hours), after the first addition, the temperature of the system was raised to 65°C, and it was controlled at 65°C during the second and third additions;
[0025] After the addition, the reaction was incubated for 3 hours, followed by TLC (TLC conditions: chloroform:methanol=6:1, volume). After the reaction is complete, cool down to 20°C, add 20ml of glacial acetic acid, control the internal temperature to not exceed 25°C, and pH=5. The solvent was evaporated under reduced pressure. 30 ml of water was added to the residue, followed by extraction with 300 ml of dichloromethane. The dichloromethane layer was washed 3 times with 30 ml of water each time....
Embodiment 3
[0027] (R)-9-[2-(Diethylphosphonomethoxy)propyl]-adenine (PMPA diethyl ester)
[0028] Under nitrogen protection, in all the solutions obtained according to the method of Example 1, 20.0 g of di-n-butyl magnesium was added, and then 40.0 g of diethyl p-toluenesulfonyloxymethyl phosphate was added dropwise (adding in three times, each time interval of 1 hour), the temperature of the system was raised to 35°C after the first addition, and was controlled at 35°C during the second and third additions; after the addition, the temperature was kept for 6 hours, and the reaction was tracked by TLC. After the same post-treatment as above, 21.2 g of orange oil was obtained, the purity of which was analyzed by area-normalized HPLC was 81%, and the yield was 67.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com