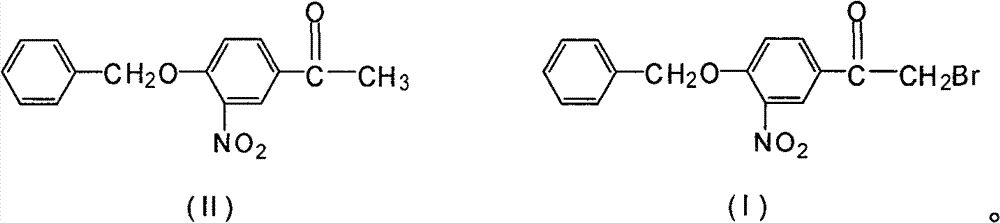
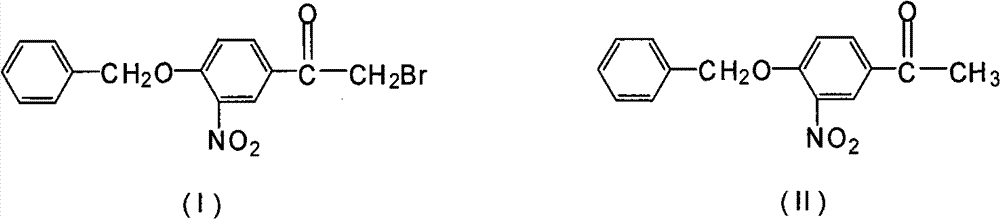
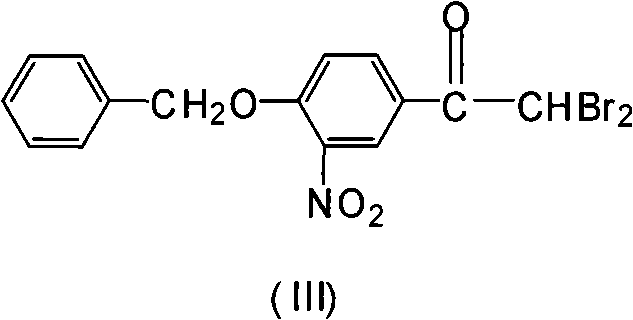
Novel synthesis method for 3-nitryl-4-benzyloxy-alpha-bromoacetophenone
A technology of benzyloxyacetophenone and bromoacetophenone, which is applied in the field of synthesis of 3-nitro-4-benzyloxy-α-bromoacetophenone, can solve the difficulties in production operation and the high toxicity of acetonitrile , Difficult to deal with and other problems, to achieve the effect of controlling the by-product dibromo, short reaction time, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 500ml three-neck flask equipped with a stirrer, dropping funnel and thermometer, add 200ml of methanol, add 54.2g of 3-nitro-4-benzyloxyacetophenone and 2.7g of phosphorus tribromide under stirring, and stir to make the solid Dissolve, keep the temperature at 5°C, start to drop the mixed solution of bromine and methanol (mixed by bromine: methanol = 1:3 (mass ratio)), react at this temperature for 1 hour, the reaction is complete, use 36g of bromine, After the reaction, the precipitate in the reaction was obtained by suction filtration, rinsed with 100 ml of petroleum ether, and baked at 60° C. for 2 hours to obtain 60.6 g with a yield of 86.6%.
Embodiment 2
[0022] In a 500ml three-neck flask equipped with a stirrer, dropping funnel and thermometer, add 200ml of methanol, add 54.2g of 3-nitro-4-benzyloxyacetophenone and 1.7g of phosphorus trichloride under stirring, and stir to make the solid Dissolve, keep the temperature at 5°C, start to drop a mixed solution of bromine and methanol (mixed by bromine: methanol=1:3 (mass ratio)), react at this temperature for 2 hours, the reaction is complete, use 45g of bromine, After the reaction, the precipitate in the reaction was obtained by suction filtration, rinsed with 100 ml of petroleum ether, and baked at 60° C. for 2 hours to obtain 53.5 g with a yield of 76.4%.
Embodiment 3
[0024] Add 200ml of methylene chloride in a 500ml three-neck flask equipped with a stirrer, dropping funnel and thermometer, add 54.2g of 3-nitro-4-benzyloxyacetophenone and 2.7g of phosphorus tribromide under stirring, and stir The solid was dissolved, and the temperature was maintained at 0° C., and a mixed solution of bromine and dichloromethane (mixed by bromine:dichloromethane=1:3 (mass ratio)) was started to be added dropwise, and the reaction was completed after 1 hour of reaction at this temperature. Use 33g to remove bromine. After the reaction, suction filter to obtain the precipitate in the reaction. After rinsing with 100ml of petroleum ether, bake at 60°C for 2 hours to obtain 59.8g with a yield of 85.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com