Dosage form for providing ascending dose of drug
A drug and dose technology, applied in drug delivery, drug combination, drug formulation, etc., can solve problems such as inability to overcome inherent defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
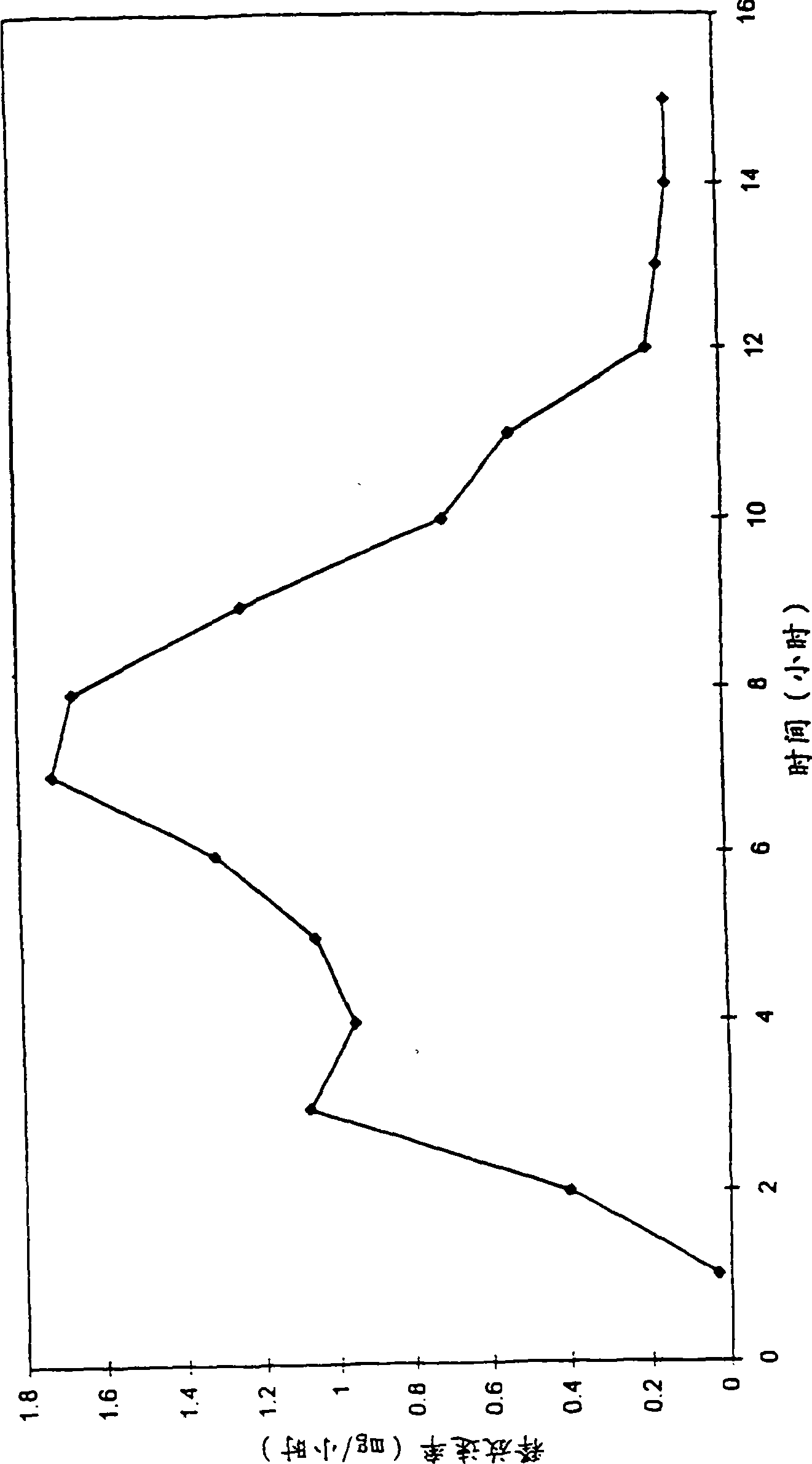
[0049] A dosage form designed and adapted for administration with an increasing release rate profile is prepared according to this example.
[0050] First, the composition of the first layer containing the drug dose was prepared as follows. 157.8 g of polyethylene oxide having a number average molecular weight of 200,000 was passed through a No. 40 mesh U.S. sieve and placed in the bowl of a conventional planetary mixer. Next, 31.2 g of methylphenidate drug was added to the blender. Then, 10 g of hydroxypropylmethylcellulose with a number average molecular weight of 9200 passed through a 40-mesh sieve and added to a mixer containing methylphenidate and polyethylene oxide. Thereafter, 0.5 g of FD&C Blue Dye No. 1 for identification was added to the drum of the blender. The ingredients were blended in a blender for 10 minutes to obtain a homogeneous composition. Next, under continuous stirring, about 100 ml of denatured absolute ethanol was gradually added to the mixer over a...
Embodiment 2
[0057] This example is prepared according to the method of Example 1, the difference is that the first layer and the second layer of drugs in this example are selected from amphetamine, dextroamphetamine, methamphetamine, ethylphenidate, amphetamine and phenetamine English, wherein the dose of the drug in the second layer is greater than the dose of the same drug in the first layer, the first layer contains 10ng to 300mg of drug and the second layer has a drug dose of 50mg to 500mg.
Embodiment 3
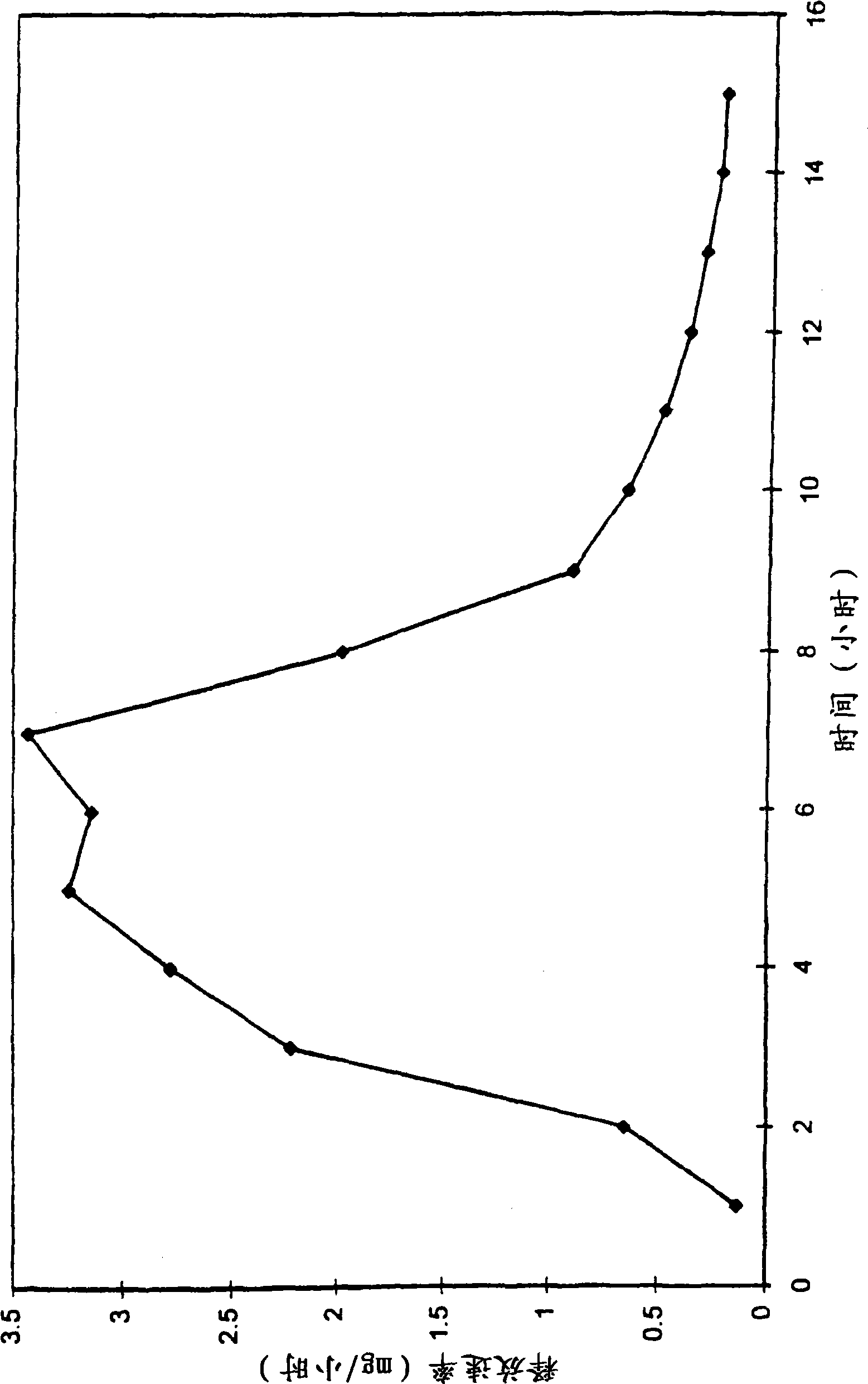
[0059] A dosage form was prepared according to this example which was designed and adapted for the administration of pseudoephedrine hydrochloride with a gradually increasing release rate profile.
[0060]First, the pharmaceutical composition of the first layer is prepared as follows. 139g of polyethylene oxide with a number average molecular weight of 300,000 passed through a 40-mesh sieve, and the first sieved hydrophilic polymer was put into the drum of the mixer. Next, 40 g of pseudoephedrine hydrochloride was added to the blender and the components were blended for 5 minutes. Then, 10 g of hydroxypropylmethylcellulose with a number average molecular weight of 9200 and 10 g of polyoxyethylene stearate 40 were passed through a 40-mesh sieve and added to a blender filled with the above components. Thereafter, all components were stirred together for 10 minutes to obtain a homogeneous composition. Next, under continuous stirring, about 100 ml of denatured absolute ethanol w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com