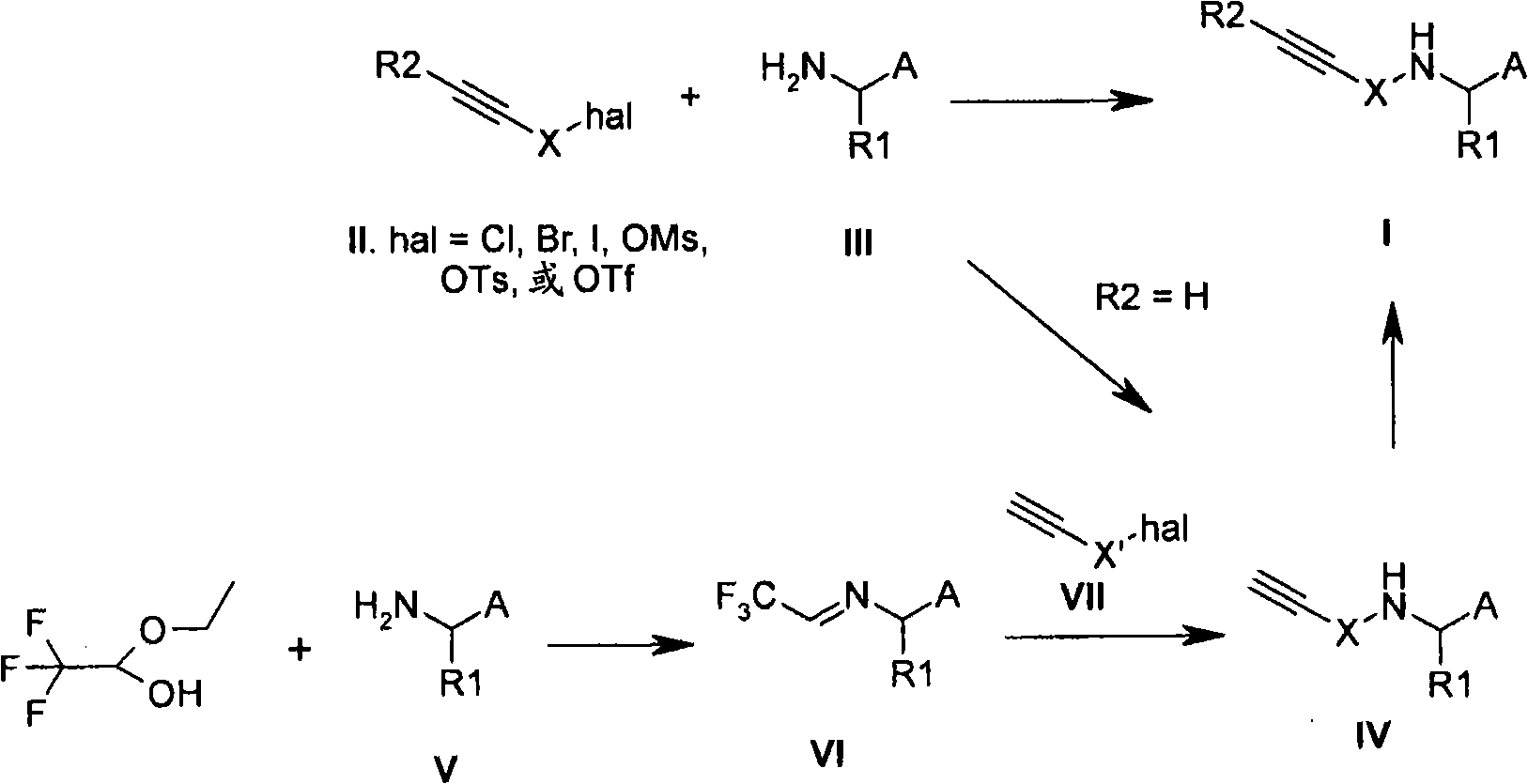
Substituted acetylenic compounds useful for the treatment of diseases
A technology of compounds and solvates, applied in the field of preparation of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0497]
[0498] (R)-But-2-ynyl-(1-naphthalen-1-yl-ethyl)-amine hydrochloride (compound 101)
[0499]1-Bromo-but-2-yne (0.66 g, 5.0 mmol) and (R)-1-naphthalen-1-yl-ethylamine (2.0 g, 11.7 mmol) were treated as described in General Method 1. The crude product was purified by chromatography (PE / EtOAc 5:1) to obtain the free amine. The free amine was redissolved in MeOH. To this solution was added aqueous hydrochloric acid (1N) until the pH was 2 and the product crystallized out. Filtration and washing of the crystals with a small amount of diethyl ether afforded the title compound as a white solid.
[0500] 13 C NMR (DMSO-d 6 ): δ=129.3, 127.2, 126.5, 125.9, 124.9, 122.9, 85.2, 70.8, 51.2, 34.8, 20.2, 3.6.
Embodiment 2
[0502]
[0503] (R)-(1-naphthalen-1-yl-ethyl)-pent-4-ynyl-amine (compound 102)
[0504] (R)-1-Naphthalen-1-yl-ethylamine (4.1 g, 24.0 mmol) and 5-chloro-pent-1-yne (2.1 g, 20.0 mmol) were dissolved in DMF (25 mL). K was added to the solution at room temperature 2 CO 3 (3.9 g, 28 mmol) and NaI (1.0 g). The reaction mixture was stirred at 50 °C for 48 h, then poured into H 2 O middle. The obtained mixture was extracted with EtOAc. The combined organic phases were subjected to MgSO 4 Dry and concentrate in vacuo. The residue was purified by chromatography (PE / EtOAc 1:1 to PE / EtOAc 0:1) to obtain the title compound as an oil.
[0505] 13 C NMR (DMSO-d 6 ): δ=130.6, 129.4, 129.3, 127.2, 126.5, 125.9, 124.9, 122.9, 85.2, 70.8, 51.2, 34.8, 20.2, 3.6.
Embodiment 3
[0507]
[0508] (E) / (R)-(6,6-Dimethyl-hept-2-en-4-ynyl-(1-naphthalen-1-yl-ethyl)-amine (compound 103) and (Z) / (R)-(6,6-dimethyl-hept-2-en-4-ynyl)-(1-naphthalen-1-yl-ethyl)-amine (compound 104)
[0509] Mix (E) / (Z)-1-bromo-6,6-dimethyl-hept-2-en-4-yne (374 mg, 2.0 mmol) and (R)-1-naphthalen-1-yl-eth The amine (342 mg, 2.0 mmol) was worked up as described in General Method 1. The crude product was purified by chromatography (PE / EtOAc 6:1) to obtain compound 103 and compound 104 as colorless oils.
[0510] Compound 103:
[0511] 13 C NMR (CDCl 3 ): δ=140.9, 140.5, 134.0, 131.3, 129.0, 127.2, 125.7, 125.7, 125.3, 123.0, 122.7, 111.3, 98.4, 77.2, 52.9, 49.3, 31.0, 27.9, 23.6.
[0512] Compound 104:
[0513] 13 C NMR (CDCl 3 ): δ=141.1, 139.8, 134.0, 131.4, 128.9, 127.2, 125.7, 125.7, 125.3, 123.0, 122.7, 111.4, 103.8, 75.2, 52.7, 46.6, 30.9, 28.0, 23.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com