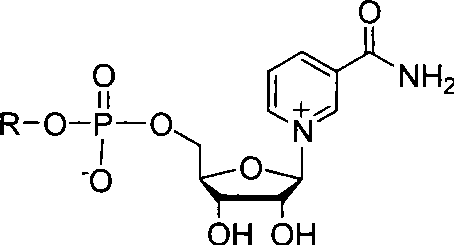
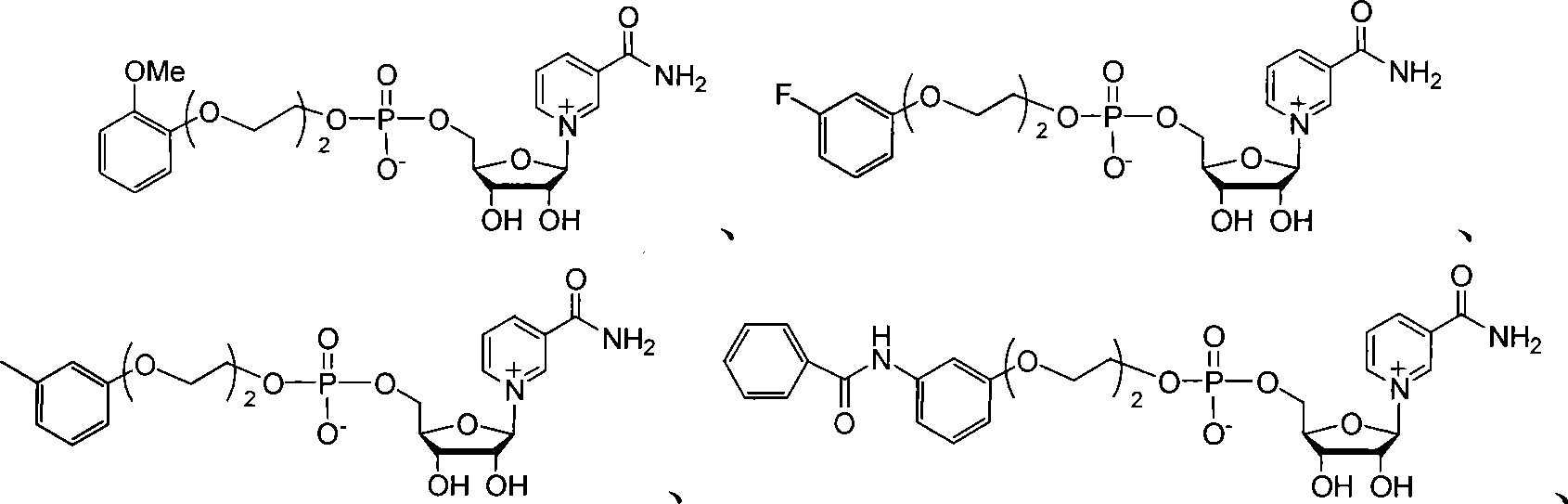
NAD+ analogue, as well as synthesis and use thereof
A technology of analogs and synthetic methods, applied in sugar derivatives, adding compounds to stimulate growth, organic chemistry, etc., can solve the problems of simple structure of cofactors, many by-products, difficult separation, etc., and achieve simple and easy separation and purification , mild reaction conditions, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 0.63mmol Ac 2 NMN Dissolve in 7ml pyridine and 7ml DMF mixed solvent, add 2-phenylethanol (200μl, 1.67mmol), add 2,4,6-triisopropylbenzenesulfonyl chloride (TPS-Cl) (757mg, 2.5mmol), 10 The reaction was stirred at °C for 10 h, the solvent was removed under reduced pressure, 10 ml of water was added dropwise, washed with dichloromethane, the aqueous phase was concentrated, and the product was collected by reverse-phase silica gel column chromatography, and concentrated to obtain a yellow syrupy solid.
[0037] The above product was dissolved in 0.7ml methanol, cooled to -5°C, and 7M NH was added. 3 115 μl of methanol solution of 115 μl, the mixture was stirred at -5 °C overnight, TLC detected until the reaction was complete, the solvent was removed, 1 ml of water was added to the residue, and the product was collected by reverse-phase silica gel column chromatography, concentrated, and then passed through anion exchange resin (201× 4. HCO 2 - type), concentrated, ly...
Embodiment 2
[0051] With the method of embodiment 1; the difference with embodiment 1 is that the alcohol used is The reaction temperature was 30°C, the reaction time was 38h, the condensation reagent used was 1-p-nitrobenzenesulfonic acid-1,2,4-triazole, and the molar ratio of pyridinium salt, alcohol and condensation reagent was 1:1:5, Yield: 59%.
[0052]
[0053] 1 H NMR (400MHz, D 2 O): δ 0.75(t, 2H), 1.23(q, 2H), 1.44(t, 2H), 3.75(q, 2H), 4.03(d, J=5.32Hz, 1H), 4.20(d, J= 10.28Hz, 1H), 4.42(t, J=5.08Hz, 1H), 4.51(s, 1H), 6.11(d, J=5.12Hz, 1H), 9.01(d, J=6.04, 1H), 8.88( d, J=7.56Hz, 1H), 8.18 (t, J=7.44Hz, 1H), 9.34 (s, 1H).
[0054] 13 C NMR (100MHz, D 2 O): δ 168.2, 148.4, 144.9, 142.2, 136.4, 130.9, 102.3, 89.7, 80.2, 73.5, 68.7, 66.8, 34.4, 20.8, 15.4.
[0055] 31 P NMR (160MHz, D 2 O): δ 0.57.
[0056] HRMS: calcd for C 15 H 23 N 2 O 8 P(M+H + )391.1230, found(M+H + )391.1244.
[0057] The compound has a molecular weight of 390.12, has UV absorption at 266 n...
Embodiment 3
[0065] With the method of embodiment 1; the difference with embodiment 1 is that the alcohol used is The reaction temperature is 35°C, the reaction time is 38h, the molar ratio of pyridinium salt, alcohol and condensation reagent is 1:3:8, the volume ratio of DMF:Py=1:8, the yield: 12%.
[0066]
[0067] 1 H NMR (400MHz, D 2 O): δ 1.20(t, 2H), 1.28(d, 6H), 1.42(d, 3H), 1.51(d, 3H), 1.69(s, 3H), 3.74(q, 2H), 4.01(dd, J=4.92, 10.4Hz, 1H), 4.17(dd, J=3.96, 9.92Hz, 1H), 4.28(s, 1H), 4.43(s, 1H), 4.48(s, 1H), 6.09(d, J = 5.04, 1H), 8.16 (t, J=7.04, 1H), 8.87 (d, J=8.04, 1H), 9.14 (d, J=6.2, 1H), 9.32 (s, 1H).
[0068] 13 C NMR (100MHz, D 2 O): δ 167.5, 148.4, 144.7, 141.9, 141.2, 136.2, 131.6, 131.0, 130.8, 128.9, 65.3, 45.7, 42.6, 38.2, 31.6, 29.3, 19.8.
[0069] 31 P NMR (160MHz, D 2 O): δ 0.63.
[0070] HRMS: calcd for C 23 H 33 N 2 O 8 P(M+H + )497.2210, found(M+H + )497.2254.
[0071] The compound has a molecular weight of 496.22, has UV absorption at 266 n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com