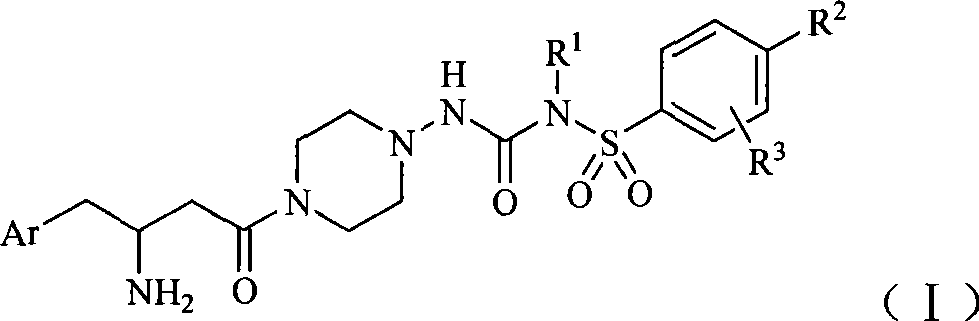
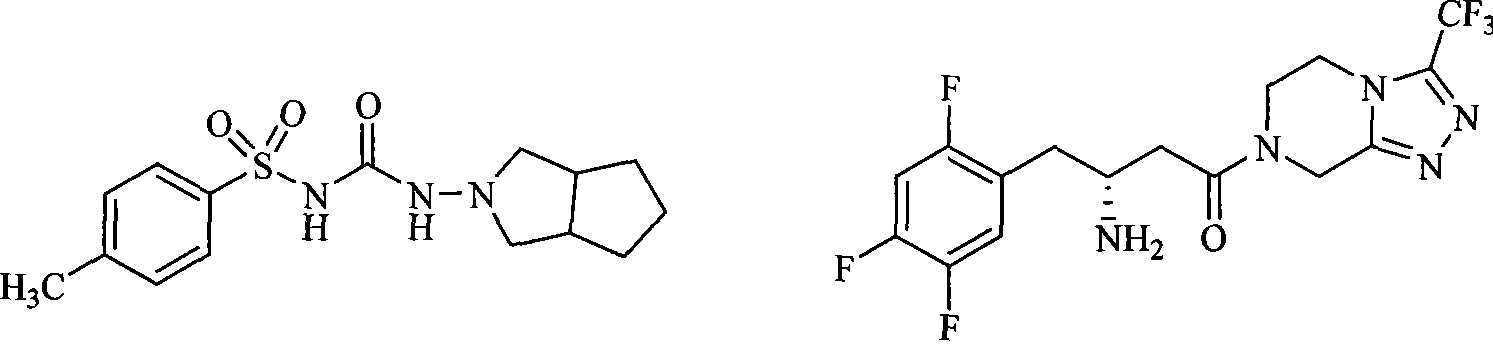
DPP-IV inhibitor with sulfonamide formamide piperazine structure
A technology based on alkyl and cyano groups, which can be used in medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc., and can solve problems such as limited drug varieties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1 (2R, 5S)-2,5-dihydro-3,6-dimethoxy-2-[(2,4,5-trifluorophenyl)methyl]-5-isopropylpyridine Preparation of oxazine
[0127] 9.2g (50mmol) (2S)-(+)-2,5-dihydro-3,6-dimethoxy-2-isopropylpyrazine was dissolved in 300mL tetrahydrofuran, and then heated at -78°C within 30min Add 24mL (60mmol, 2.5N) of n-butyllithium / n-hexane solution dropwise, and keep stirring for 30min. Then add dropwise 9.9g (55mmol) of 2,4,5-trifluorobenzyl chloride in 50mL of tetrahydrofuran cold solution, - The reaction was stirred at 78°C for 5h. Add 100 mL of water at -78°C to quench the reaction, concentrate the reaction solution, add ethyl acetate and 1N hydrochloric acid to the residue, separate the aqueous layer, extract three times with ethyl acetate, combine the organic layers, wash with salt, dry over anhydrous magnesium sulfate, reduce Concentrate under reduced pressure and purify on a silica gel column (the eluent is a mixture of ethyl acetate and cyclohexane) to obtain 12 g of th...
Embodiment 2
[0128] Example 2 Preparation of (R)-N-2-tert-butoxycarbonyl-3-(2,4,5-trifluorophenyl)alanine methyl ester
[0129] 16.4g (50mmol) of (2R,5S)-2,5-dihydro-3,6-dimethoxy-2-[(2,4,5-trifluorophenyl)methyl]-5-iso Propylpyrazine was dissolved in 100mL of acetonitrile, then 100mL of 1N hydrochloric acid was added dropwise, and the reaction solution was stirred at room temperature for 24h. Methanol was added, concentrated to dryness, this operation was repeated three times, and then repeated once with toluene to obtain a solid. After the solid was dissolved in 400mL of dichloromethane, 50g of triethylamine was slowly added, and then 24g (110mmol) (Boc) was slowly added dropwise. 2 O. The reaction solution was stirred at room temperature for 8 h, and the solid generated by the reaction was filtered off. The filtrate was diluted with dichloromethane, washed with 1N HCl solution and saturated brine respectively, dried over anhydrous magnesium sulfate, purified on a silica gel column, ...
Embodiment 3
[0130] Example 3 Preparation of (R)-N-2-tert-butoxycarbonyl-3-(2,4,5-trifluorophenyl)alanine
[0131] Add 200mL tetrahydrofuran to 10g (30mmol) of (R)-N-2-tert-butoxycarbonyl-3-(2,4,5-trifluorophenyl)alanine methyl ester, cool in an ice bath, and then dropwise add 21.5 g (90mmol) lithium hydroxide aqueous solution 200mL, the reaction solution was stirred at room temperature for 15h. Concentrate under reduced pressure, and dissolve the residue with ethyl acetate, wash with saturated sodium bicarbonate and brine, and dry over anhydrous magnesium sulfate. The solvent was distilled off to obtain 7.7 g of solid, yield: 80.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com