Preparation of glimepiride raw material
A glimepiride and raw material technology, applied in the field of preparation of glimepiride raw materials for the treatment of type II diabetes, can solve the problems of high content of organic solvent chloroform and difficult removal, etc., so as to be suitable for industrial production and reduce residues , the effect of simplifying the synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
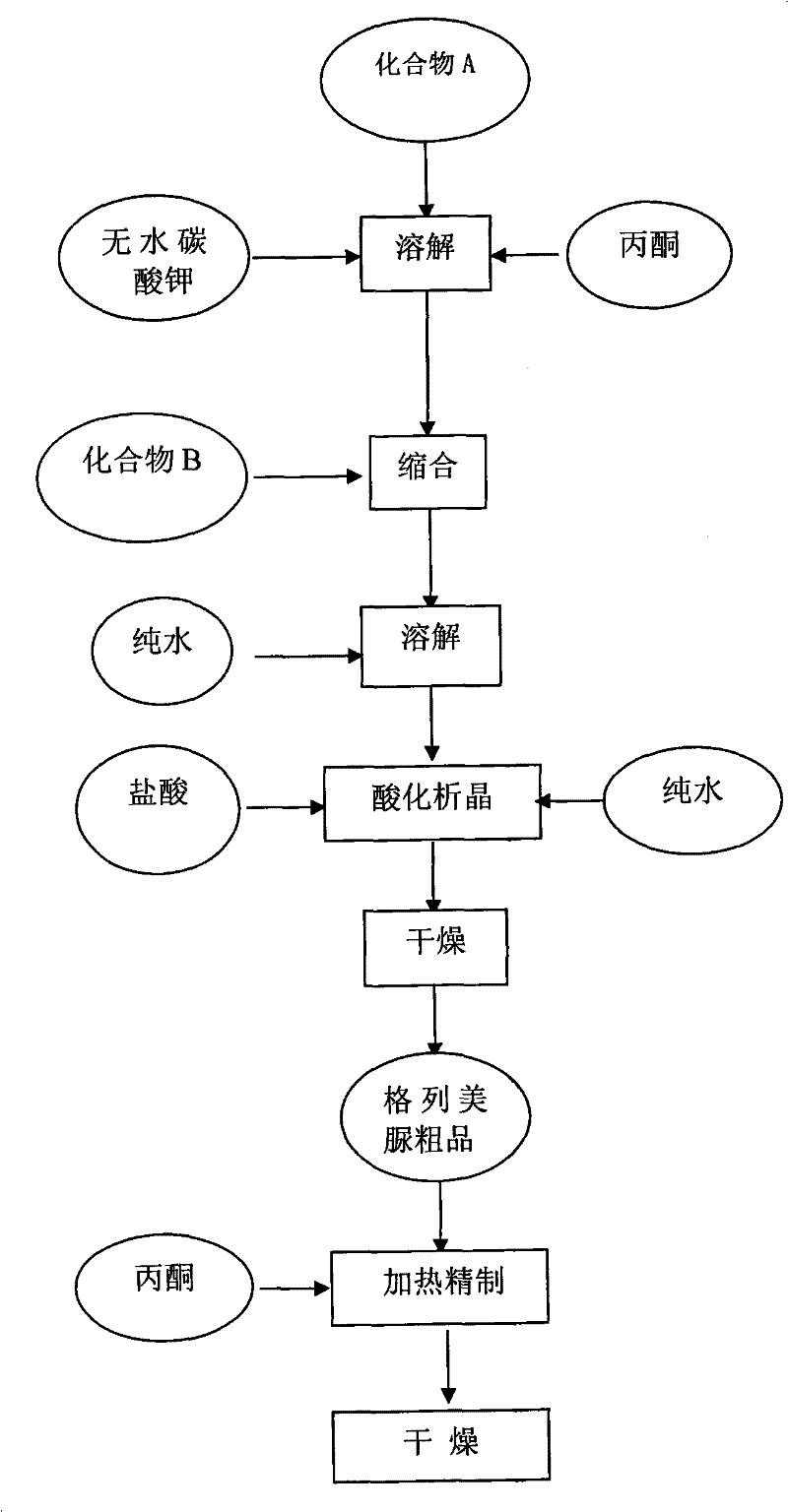
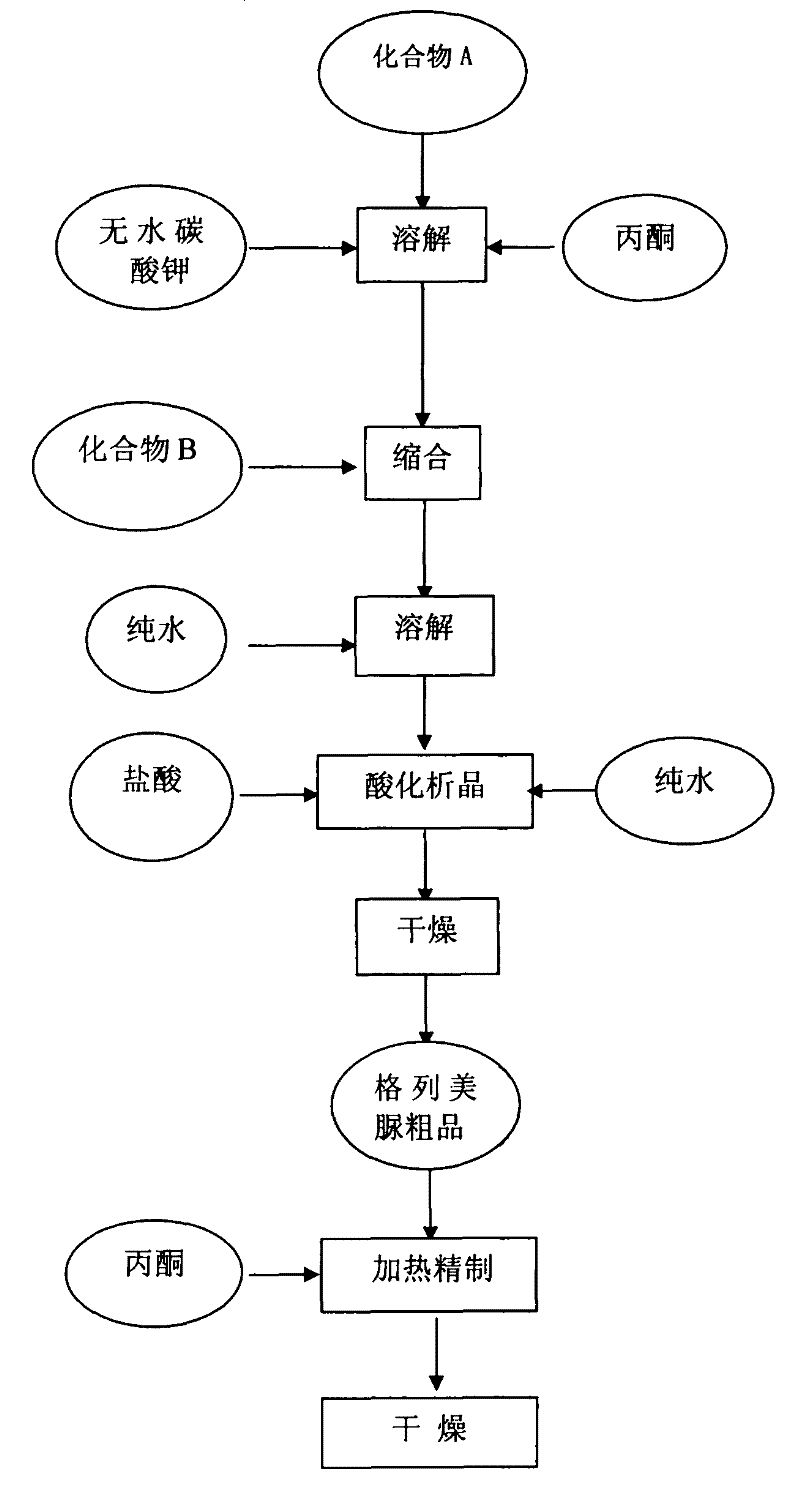
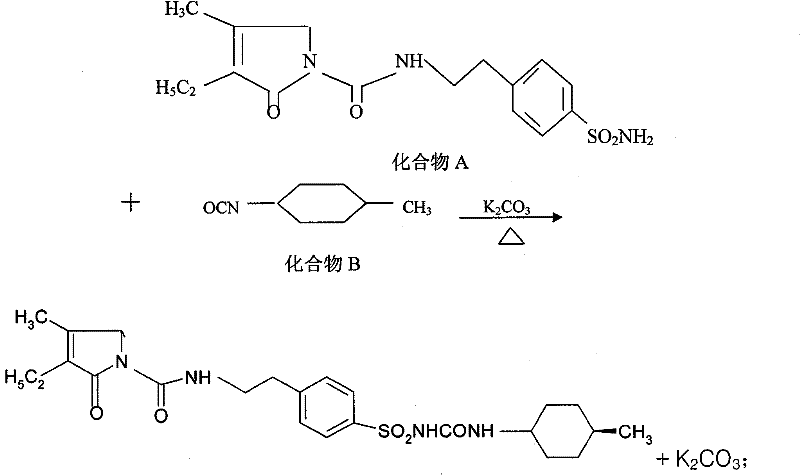
[0027] A kind of preparation method of glimepiride raw material, with compound A: 4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-formylamino)-ethyl ]-benzenesulfonamide and compound B: trans-4-methyl-cyclohexyl isocyanate is raw material, comprises the following steps in order:
[0028] The first step: Add compound A: 4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-formylamino) in the reaction kettle according to 1:0.81:20 -Ethyl]-benzenesulfonamide, anhydrous potassium carbonate and organic solvent acetone, heated to 56°C and refluxed for 5 hours under stirring, then stopped heating; when the temperature was lowered to 50°C, slowly added compound B: trans-4-methyl - cyclohexyl isocyanate, wherein, the ratio of benzenesulfonamide and trans-4-methyl-cyclohexyl isocyanate addition is 1: 0.43 (molar ratio is about 1: 1); Continue heating and stirring to reflux at this temperature for 5.5 hours, Stop heating and let it stand for more than 8 hours to obtain a synthetic liquid of a mixture ...
Embodiment 2
[0034] A kind of preparation method of glimepiride raw material, with compound A: 4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-formylamino)-ethyl ]-benzenesulfonamide and compound B: trans-4-methyl-cyclohexyl isocyanate is raw material, comprises the following steps in order:
[0035] Step 1: Add compound A: 4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido) in the reaction kettle according to 1:0.81:22 -Ethyl]-benzenesulfonamide, anhydrous potassium carbonate and organic solvent acetone, heated to 60°C and refluxed for 5.5 hours under stirring, then stopped heating; when the temperature was lowered to 54°C, slowly added compound B: trans-4-methyl - Cyclohexyl isocyanate, wherein, the ratio of benzenesulfonamide and trans-4-methyl-cyclohexyl isocyanate addition is 1:0.43 (molar ratio is about 1:1); Continue to stir and reflux at this temperature for 5.5 hours, stop Heating and standing for more than 8 hours to obtain a synthetic liquid of a mixture of crude glimepiride and...
Embodiment 3
[0040] A kind of preparation method of glimepiride raw material, with compound A: 4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-formylamino)-ethyl ]-benzenesulfonamide and compound B: trans-4-methyl-cyclohexyl isocyanate is raw material, comprises the following steps in order:
[0041] The first step: Add compound A: 4-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-formylamino) in the reaction kettle according to 1:0.81:20 -Ethyl]-benzenesulfonamide, anhydrous potassium carbonate and organic solvent acetone, heated to 56°C and refluxed for 6 hours under stirring, then stopped heating; when the temperature was lowered to 50°C, slowly added compound B: trans-4-methyl - Cyclohexyl isocyanate, wherein, the ratio of benzenesulfonamide and trans-4-methyl-cyclohexyl isocyanate is 1:0.43 (molar ratio is about 1:1); continue to stir and reflux at this temperature for 6 hours, stop Heating and standing for more than 8 hours to obtain a synthetic liquid of a mixture of crude glimepiride and potass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com