Pharmaceutical use of triptolide
A technology of triptolide and tripterygium, which is applied in the field of preparation of anti-hematological malignancy drugs, can solve the problems of unseen research reports, hormone resistance and sensitivity reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 Steps and methods of mechanism discovery
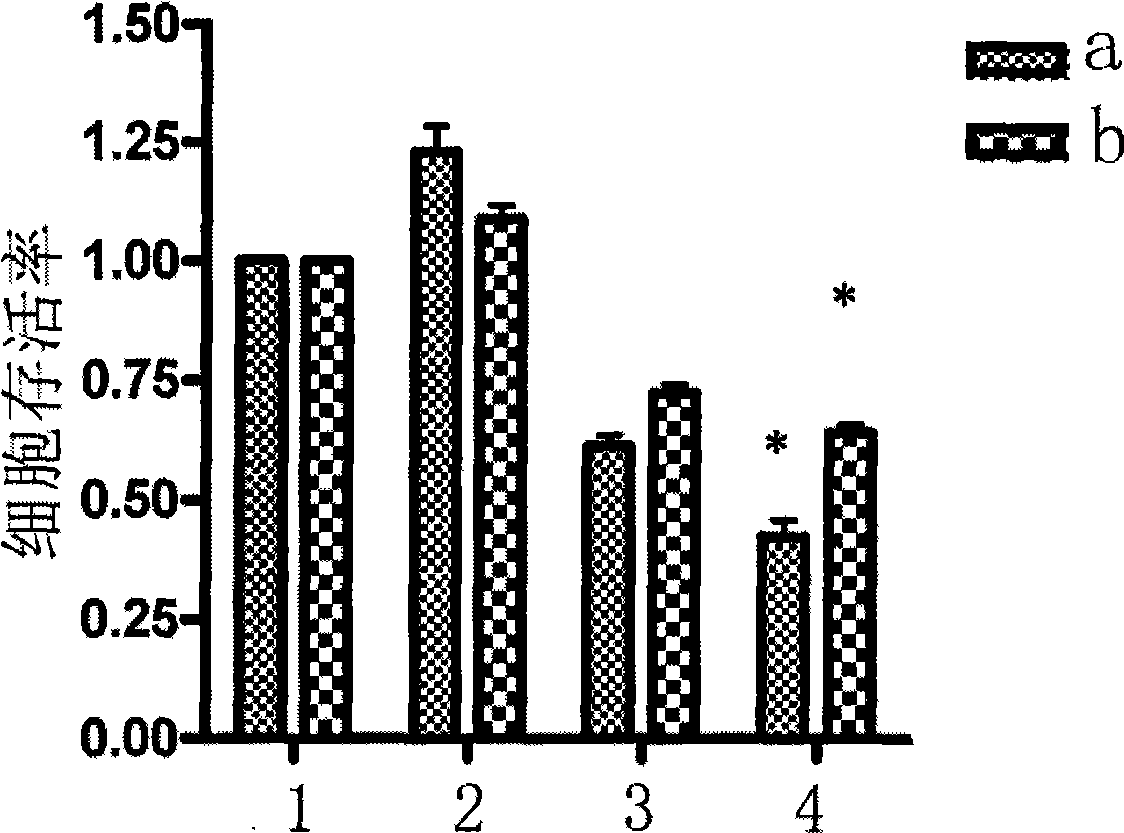
[0016] We studied the effect of IL-6 on TPL-induced dexamethasone-sensitive and drug-resistant multiple myeloma cell lines MM.1S, MM.1R. The results of MTT colorimetric assay showed that 50ng / ml IL-6 could promote the growth of MM.1S and MM.1R cells, and the cell survival rates were 123±3.64% and 109±5.77%, respectively. 5ng / ml TPL treated MM.1 cells for 24h alone and in combination with IL-6 (50ng / ml), IL-6 could not antagonize TPL-induced growth inhibition of MM.1 cells, the cell survival rate was 42.33±2.36%, 64 ±3.17%, 61±2.08% and 72.33±3.98% when combined with IL-6. There were significant differences among the groups (Pfigure 1 . In the figure a is the dexamethasone-sensitive multiple myeloma cell line MM.1S, b is the dexamethasone-resistant multiple myeloma cell line MM.1R, 1 is the control, and 2 is the interleukin 650ng / ml treatment group , 3 is the triptolide 10ng / ml dose treatment group, 4 is the comb...
Embodiment 2
[0046] Effect of IL-6 on TPL-induced dexamethasone-sensitive and drug-resistant multiple myeloma cell lines MM.1S, MM.1R.
[0047] We studied the effect of IL-6 on TPL-induced dexamethasone-sensitive and drug-resistant multiple myeloma cell lines MM.1S, MM.1R. The results of MTT colorimetric assay showed that 50ng / ml IL-6 could promote the growth of MM.1S and MM.1R cells, and the cell survival rates were 123±3.64% and 109±5.77%, respectively. 5ng / ml TPL treated MM.1 cells for 24h alone and in combination with IL-6 (50ng / ml), IL-6 could not antagonize TPL-induced growth inhibition of MM.1 cells, the cell survival rate was 42.33±2.36%, 64 ±3.17%, 61±2.08% and 72.33±3.98% when combined with IL-6. There were significant differences among the groups (Pfigure 1 (Same diagram as above).
[0048] MTT detection MTT colorimetric method to detect cell proliferation:
[0049] Take cells in logarithmic growth phase, wash with fresh culture medium, resuspend in RPMI1640 culture medium co...
Embodiment 3
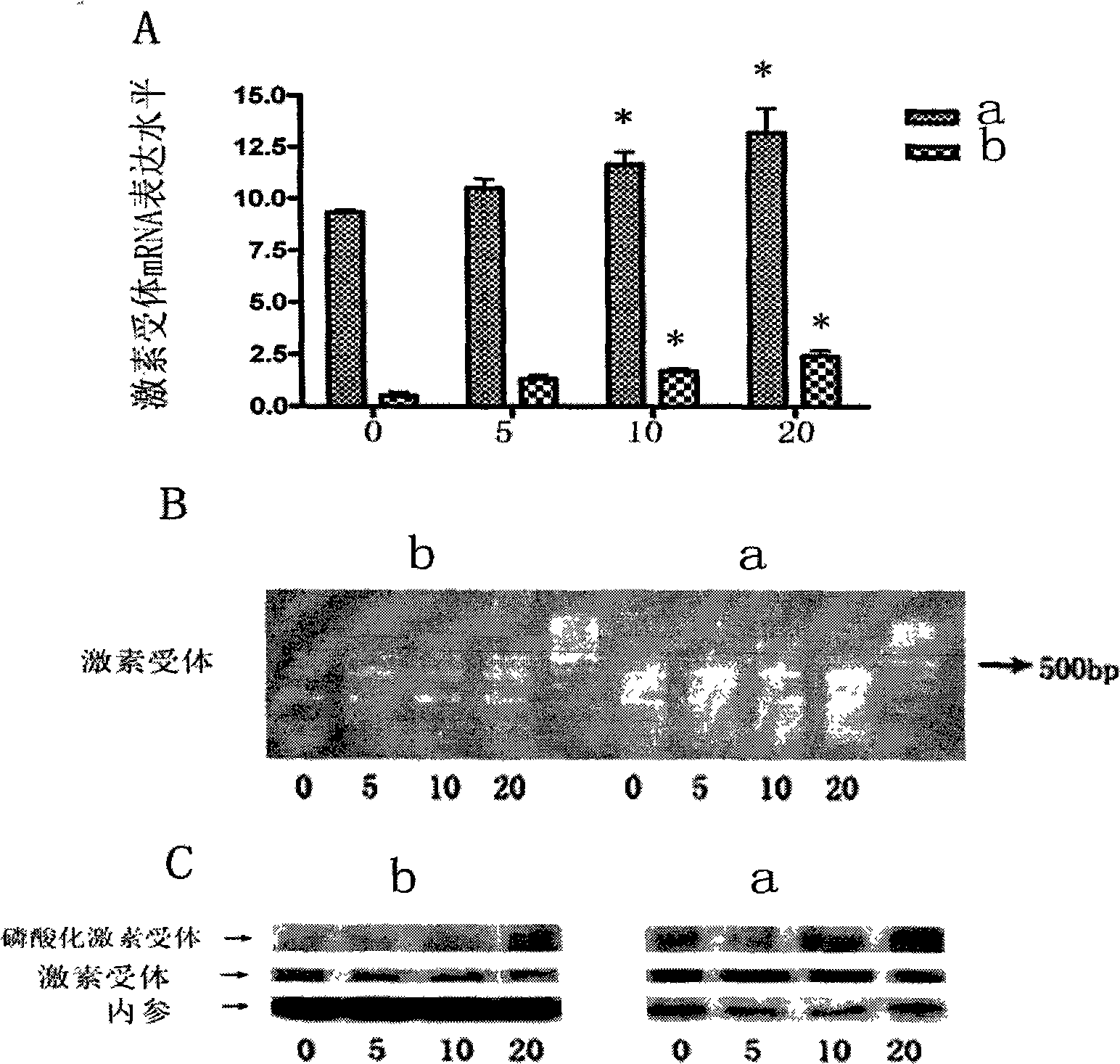
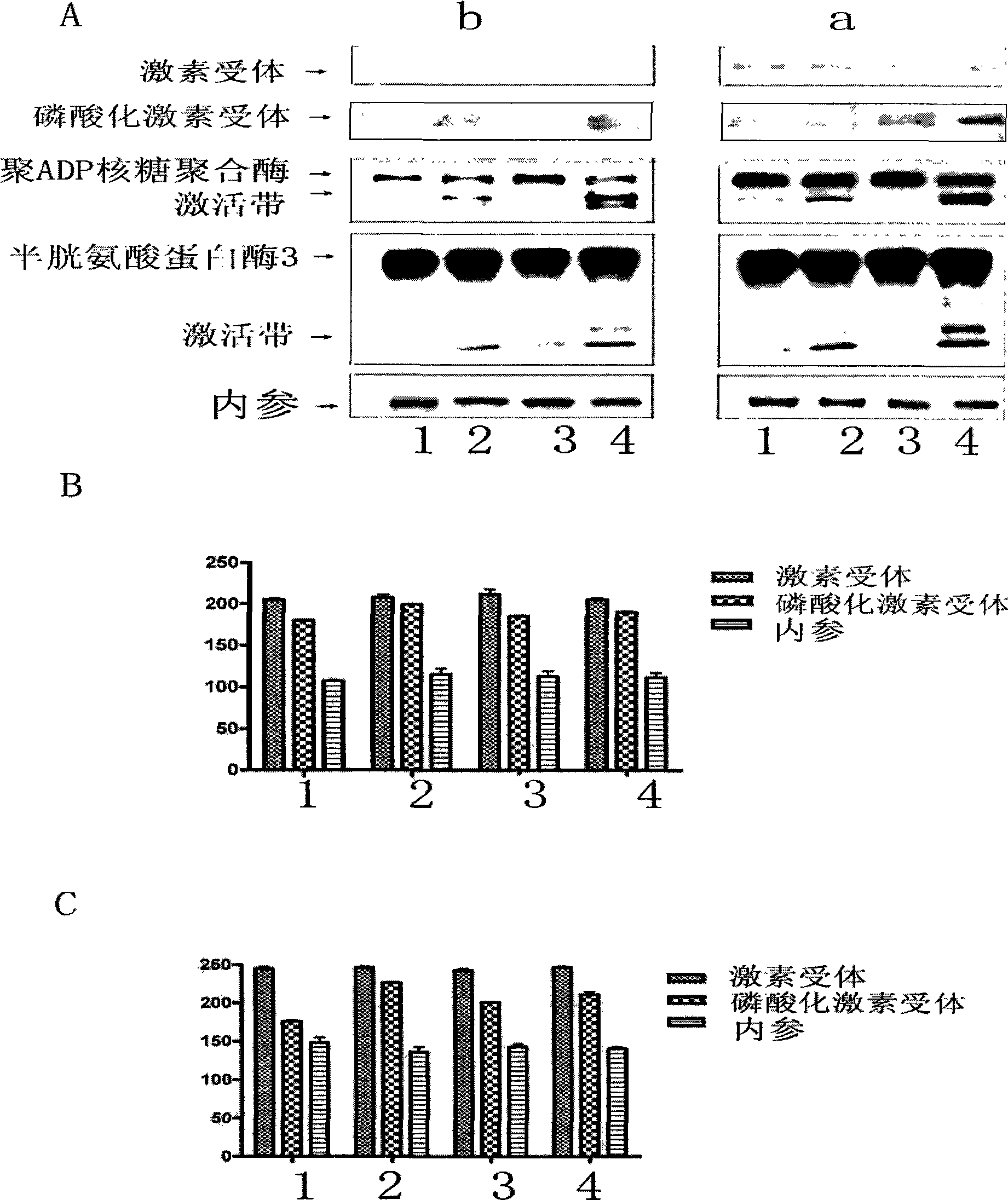
[0051] We applied real-time quantitative PCR method, common PCR method, and Western-blot method to detect the expression of GR in MM.1 cells treated with TPL. The results showed that 5-20ng / ml TPL treatment of MM.1 cells caused GR to The expression level and protein level increase, and the characteristics of the increase are also positively correlated with the apoptosis events mediated by the caspase family. For the relative expression of the target protein, see image 3 .
[0052] figure 2 The results showed that 5-20ng / mlTPL treatment of MM.1 cells caused an increase in the expression of GR at the mRNA level and protein level. figure 2 A is Real-time PCR method detection, figure 2 B is the result detected by common PCR method, figure 2 C is the increase in the expression of hormone receptors at the protein level detected by Western-blot method. In the figure, 0, 5, 10, and 20 are the treatment concentrations of triptolide, and the unit is ng / ml, * indicates that the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com