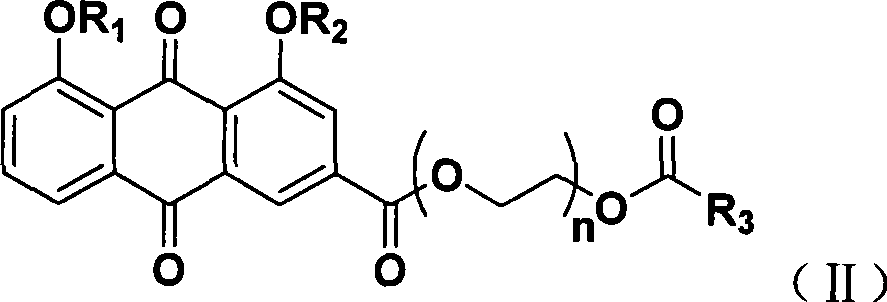
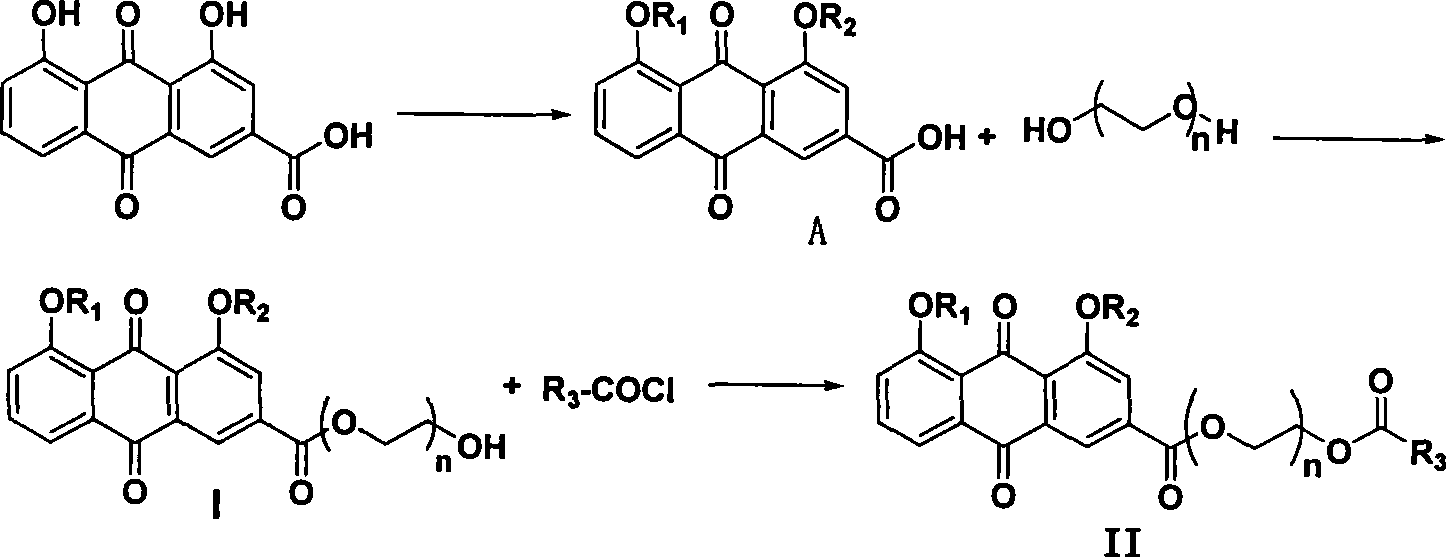
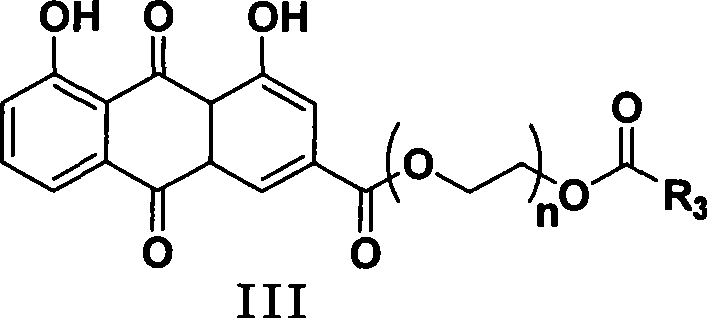
Synthesizing method and use of bone-targeted antiphlogistic medicament
A reaction and compound technology, applied in the field of medicine, can solve problems such as affecting drug absorption, low solubility, and limiting clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Synthesis of 4,5-diacetyl-anthraquinone-2-acid-2'-hydroxyethyl ester (I-1); (4,5-diacetyl-anthraquinone-2-acid-2 '-hydroxyethyl ester)
[0053] Step a: Add 50 ml of dry dichloromethane, 2.0 g (9.693 mmol) of DCC and 10 drops of dry DMF into a 100 ml eggplant-shaped bottle, and stir at room temperature for 0.5 hours. Afterwards, 2 g (5.435 mmol) of diacetylrhein was added, stirred and reacted at 30° C. for 1 hour, and then all the solvent was distilled off under reduced pressure to obtain the acid chloride. Step b: In a 250ml three-necked bottle, add 50ml of dry ethylene glycol and 1.3ml (9.250mmol) of dry triethylamine. Dissolve the acid chloride prepared in the previous step in about 150ml of dry dioxane, and slowly add it dropwise into a three-necked bottle at room temperature. After the drop is complete, stir at room temperature for 0.5 hour.
[0054] Post-treatment: Add about 2-3ml of water to the reaction solution to stop the reaction, and distill off ...
Embodiment 2
[0057] Example 2: Synthesis of 4,5-diacetyl--anthraquinone-2-acid-2'-(2-hydroxyethoxy) ethyl ester (I-2)
[0058] Step a: In a 100ml eggplant-shaped bottle, add 50ml of dry dichloromethane, 20.0g (96.93mmol) of DCC and 10 drops of dry DMF, and stir at room temperature for 0.5 hours. Afterwards, 2 g (5.435 mmol) of diacetylrhein was added, stirred and reacted at 30° C. for 1 hour, and then all the solvent was distilled off under reduced pressure to obtain the acid chloride. Step b: In a 250ml three-necked bottle, add 50ml of dry diethylene glycol and 1.5ml of dry pyridine. Dissolve the acid chloride prepared in the previous step in about 150ml of dry dioxane, and slowly add it dropwise into a three-necked bottle at room temperature. After the drop is complete, stir at room temperature for 0.5 hour.
[0059] Post-treatment: Pour the reaction solution into about 100ml of ice water, extract with dichloromethane, wash with saturated aqueous sodium chloride solution, and dry over a...
Embodiment 3
[0062] Example 3: Synthesis of 4,5-diacetyl-anthraquinone-2-acid-2'-(2-(2-hydroxyethoxy)ethoxy)ethyl ester (I-3)
[0063] Step a: In a 100ml eggplant-shaped bottle, add 10ml of dry dichloromethane, 2.0g (9.693mmol) of DCC and 10 drops of dry DMF, and stir at room temperature for 0.5 hours. Afterwards, 2 g (5.435 mmol) of diacetylrhein was added, stirred and reacted at 90° C. for 1 hour, and then all the solvent was distilled off under reduced pressure to obtain the acid chloride. Step b: In a 250ml three-necked bottle, add 50ml of dry triethylene glycol and 1.5ml of dry pyridine. Dissolve the acid chloride prepared in the previous step in about 50ml of dry THF, and slowly add it dropwise into a three-necked bottle at room temperature, after the drop is complete, stir at room temperature for 2.5 hours.
[0064] Post-treatment: Pour the reaction solution into about 100ml of ice water, extract with dichloromethane, wash with saturated aqueous sodium chloride solution, and dry ov...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com