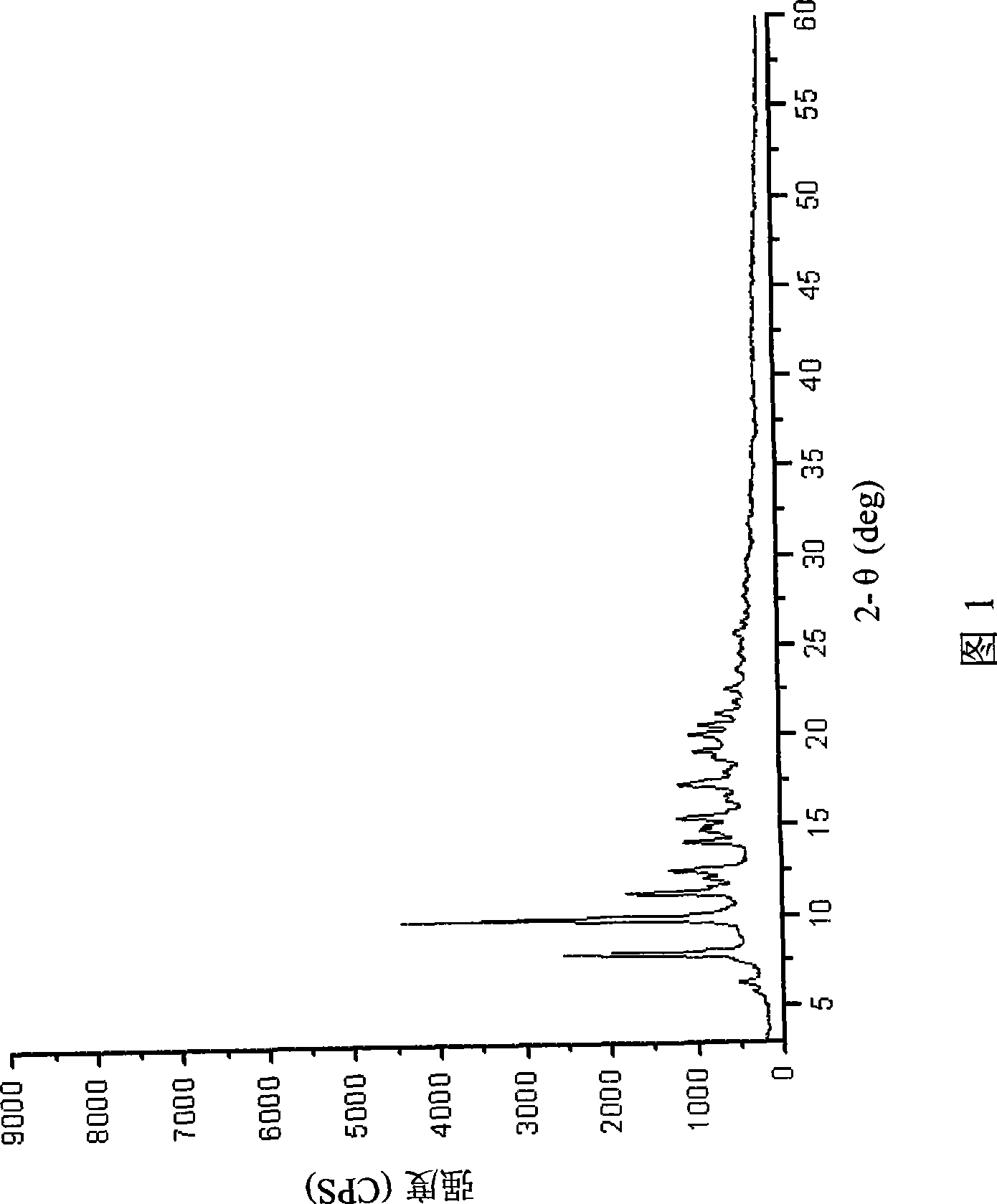
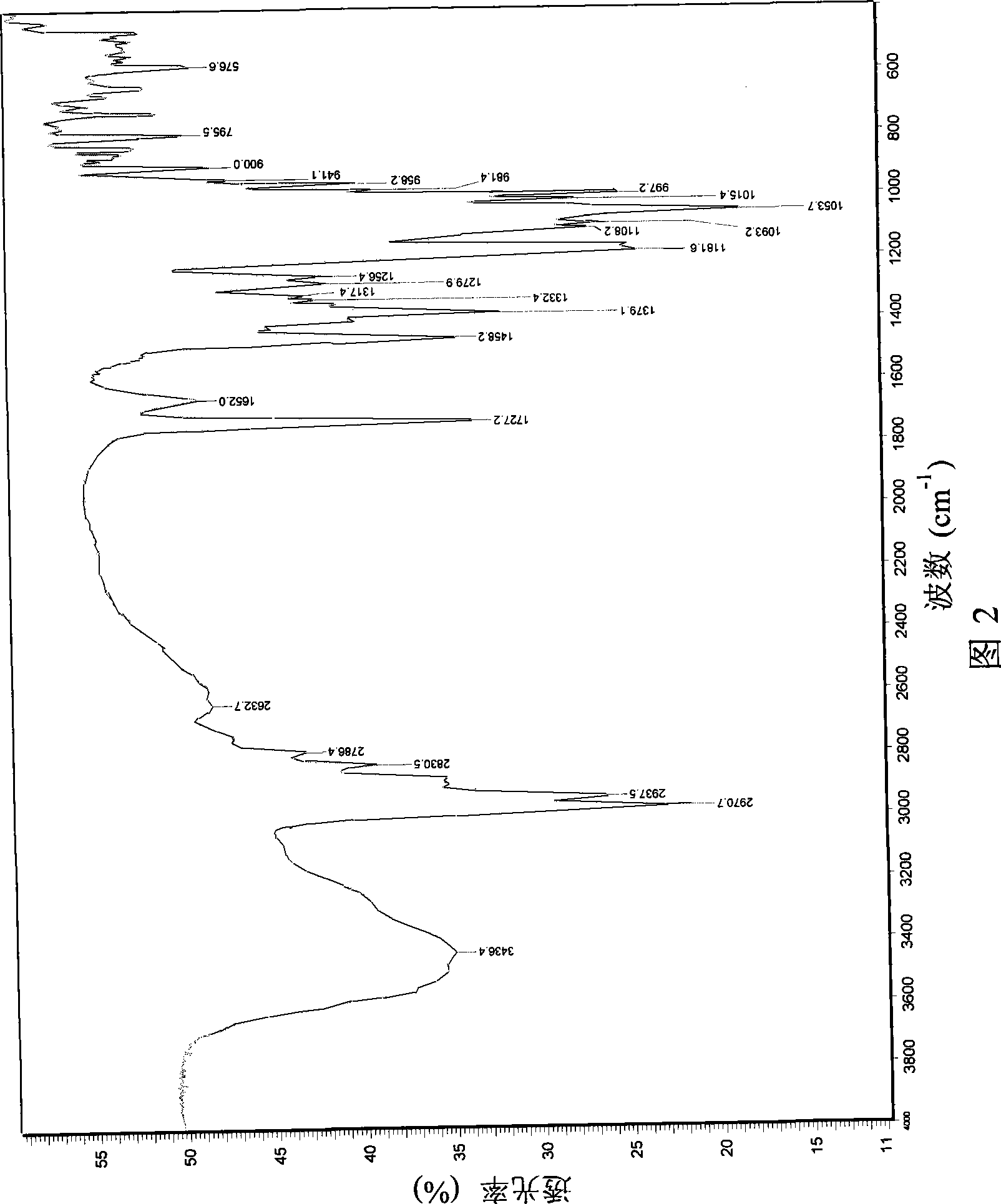
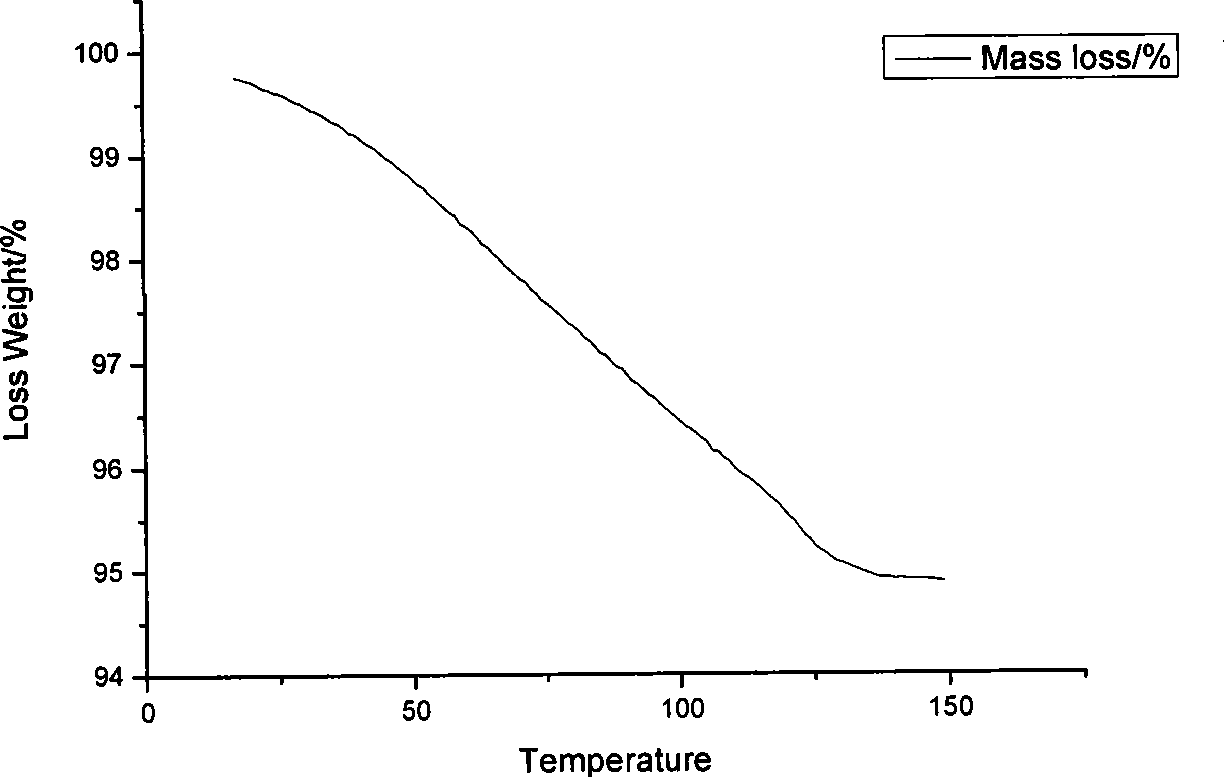
Preparation method of azithromycin monohydrate crystal
A technology for azithromycin and monohydrate, which is applied in the field of preparation of azithromycin monohydrate crystals, can solve the problems of inability to accurately control, cumbersome and difficult to operate, low azithromycin monohydrate content, etc., and achieves convenient operation, small residual solvent content, and moisture absorption. low sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The invention discloses a method for preparing crystals of azithromycin monohydrate, which comprises the following steps:
[0029] (1) Sample dissolution: dissolve the crude product of azithromycin in anhydrous methanol to obtain solution I;
[0030] (2) Crystallization: take solution I and press filter to remove insoluble matter to obtain solution II, add pure water dropwise to solution II while stirring, after the crystallization of azithromycin, centrifuge to collect the wet product of azithromycin;
[0031] (3) Pulverization: pulverize the azithromycin wet product obtained in step (2) into 60-100 meshes;
[0032] (4) Water exchange: add pure water to the pulverized azithromycin wet product obtained in step (3), and stir for 8 to 10 hours to obtain a suspension;
[0033] (5) Drying: the suspension is centrifuged to collect azithromycin crystals and dried to constant weight to obtain azithromycin monohydrate crystals.
[0034] Step (1) is carried out at 40-50°C.
...
Embodiment 1
[0049] Preheat 35kg of anhydrous methanol to 40°C, add 7kg of azithromycin, heat to 45°C, and keep warm for 1 hour. Filter to remove insoluble impurities to obtain azithromycin-methanol solution. Cool the solution to 40°C, stir quickly, add 11kg of pure water dropwise within 1 hour, keep the solution temperature at 40°C, continue to stir, and after growing the crystal for 1.5 hours, continue to add 24kg of pure water dropwise, and control it within 2 hours After the addition, continue to stir until crystallization, and grow crystals for 1.5 hours. Cool down to 23°C and keep warm for 1 hour. Filter to obtain azithromycin wet product, crush the azithromycin wet product into 60 mesh, add 70kg of pure water, stir for 8 hours, centrifuge, wash the azithromycin wet product twice with pure water, and continue to centrifuge for 5 minutes. Dry the wet product of azithromycin in a vacuum drying oven at 45°C to constant weight to obtain off-white powder, which is the crystal of azithro...
Embodiment 2
[0051]Preheat 28kg of anhydrous methanol to 40°C, add 1kg of azithromycin, heat to 50°C, and keep warm for 0.5 hours. Filter to remove insoluble impurities to obtain azithromycin-methanol solution. Cool the solution to 42°C, stir quickly, add 11kg of pure water dropwise within 1.5 hours, keep the solution temperature at 40°C, continue to stir, and after 1.5 hours of crystal growth, continue to drop 24kg of pure water within 1.5 hours After the addition, continue to stir until crystallization, and grow crystals for 1.5 hours. Cool down to 25°C and keep warm for 1 hour. Filter to obtain azithromycin wet product, crush the azithromycin wet product into 80 mesh, add 10kg of pure water, stir for 10 hours, centrifuge, wash the azithromycin wet product twice with pure water, and continue centrifuging for 5 minutes. Dry the wet product of azithromycin in a vacuum drying oven at 25°C to constant weight to obtain off-white powder, namely azithromycin monohydrate crystals, with azithro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com