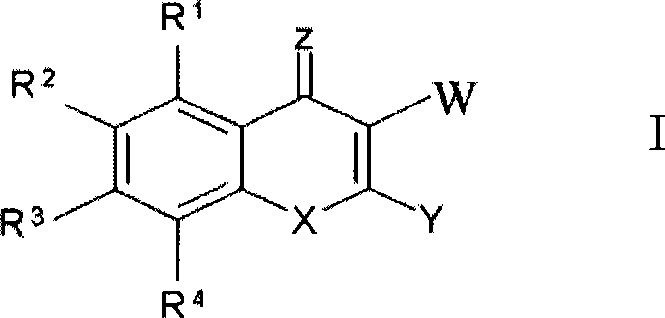
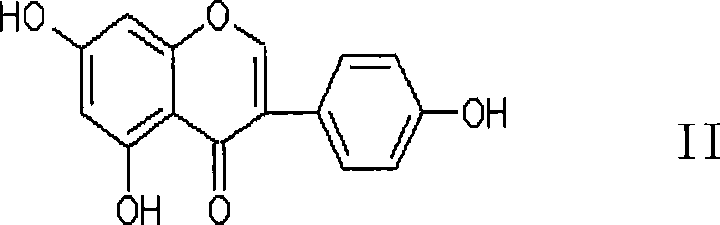
Compound and new usage of composition thereof
A compound and composition technology, applied in the field of pharmacy, can solve problems such as increasing white blood cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Synthetic routes of three genistein compounds
[0122]
[0123] Genistein analog 1 (AG-1, formula III)
[0124] The preparation method of formula III compound:
[0125] 1. In a 50ml round-bottomed three-neck flask treated with anhydrous and oxygen-free treatment, under nitrogen protection, add 25ml of newly distilled boron trifluoride ether, resorcinol (5mmol) and p-hydroxyphenylacetic acid (5mmol), oil Heating the bath to 60-90°C, stirring electromagnetically, and reacting for 8-10 hours, then cooling to room temperature;
[0126] 2. Add 8ml of anhydrous N, N-dimethylformamide, reheat to 50°C, slowly add a mixture of 1.2ml methanesulfonyl chloride and 2ml N, N-dimethylformamide, and continue heating to 60-70 ℃, react for 8-10 hours, and cool to room temperature;
[0127] 3. Pour the reaction product in batches into 100-200 ml of cooled 10% sodium acetate aqueous solution, filter, collect the solid crude product, and obtain the pure product (compound of formula II...
Embodiment 2
[0141] Therapeutic effect of genistein compounds on cyclophosphamide-induced leukopenia in mice
[0142] Experimental animals:
[0143] Eighty male SPF grade C57 BL / 6 mice were purchased from Shanghai Slack Experimental Animal Co., Ltd., weighing 22-24 g. Animals were housed in an SPF-grade animal room with 12 hours of light / 12 hours of darkness and free access to feed and water.
[0144] Experimental Drugs:
[0145]Genistein (compound of formula II), with a purity of 98%, was purchased from Nanjing Qingze Pharmaceutical Technology Development Co., Ltd.; AG-1, AG-2, and AG-3 (prepared in Example 1); Produced by Bell Pharmaceutical Co., Ltd.; Cyclophosphamide for Injection, produced by Jiangsu Hengrui Pharmaceutical Co., Ltd.
[0146] experimental method:
[0147] 1. Modeling method:
[0148] The animals were randomly divided into 8 groups. Except for the normal control group, the mice in the other groups were given intraperitoneal injection of cyclophosphamide 100 mg / kg, ...
Embodiment 3
[0166] Therapeutic effect of apigenin on cyclophosphamide-induced leukopenia in mice
[0167] Experimental animals: 60 male SPF grade C57BL / 6 mice, purchased from Shanghai Slack Experimental Animal Co., Ltd., weighing 18-22 g. Animals were housed in an SPF-grade animal room with 12 hours of light / 12 hours of darkness and free access to feed and water.
[0168] Experimental drugs: apigenin (compound of formula VI), with a purity of 98%, was purchased from Nanjing Qingze Pharmaceutical Technology Development Co., Ltd.; batyl alcohol was produced by Jiangsu Pengyao Pharmaceutical Co., Ltd.; cyclophosphamide for injection was purchased from Jiangsu Hengrui Medicine Co., Ltd. production.
[0169] experimental method:
[0170] 1. Modeling method: Animals were randomly divided into 6 groups. Except for the normal control group, mice in other groups were given intraperitoneal injection of cyclophosphamide 100 mg / kg, once a day, for 3 consecutive days to form a leukopenia model.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com